Comparison of 1st and 2nd Generation GMAF

Dr. Nobuto Yamamoto
First Generation GMAF

Dr. Hitoshi Hori
Second Generation GMAF

Dr. Yoshihiro Uto
Second Generation GMAF

Dr. Kentaro Kubo
Second Generation GMAF
Dr Yamamoto developed GMAF
Dr Yamamoto visited Dr Hori at Tokushima University, GMAF research started at Tokushima University
Dr Uto joined Dr Hori's GMAF research team
First research papers published on GMAF in the journals Biotherapy and Comparative Biochemistry & Psyhology
Tokushima University began collaborating with Saisei Mirai to develop Second Generation High Dose GMAF
Second Generation GmAF produced in our Cell Procesing Center (CPC) for patients. Start of clinical use.
Two research papers published in Antican_cer Research by Saisei Mirai & Tokushima University
Over 1000 patients treated with Saisei Mirai GMAF
New research paper published in Antican_cer Research by Saisei Mirai & Tokushima University
PROTOKOL Gc_immune_Maf - Gc macr.fag Activation Factor
Gc_immune_Maf Japanese protocol Macr.fag MODULATION (according to Dr. Akira Yamamoto) ONCOL. PATIENTS LIVE 5 TO 17 YEARS LONGER, The Japanese RECOVERY STATISTICS after five years is between 46% and 100% (depending on the stage and type of canc., as well as the patient's condition).
Prevod:
PROTOKOL Gc_immune_Maf - Gc macr.fag aktivacijski faktor
Gc_immune_Maf Japanski protokol - Macr.fag MODULACIJA (prema dr. Akiri Yamamoto) ONCOL. PACIJENTI ŽIVE OD 5 DO 17 GODINA DUŽE, Japanska STATISTIKA OPORAVKA nakon pet godina je između 46% i 100% (ovisno o stadiju i vrsti karcinoma, kao i stanju pacijenta).

Written by: Bill Sardi in Timothy Habel, October 2008
Humans get their SYSTEM at birth. The baby's body intakes positive flora from the umbilical cord and colostrum activates and awakens the SYSTEM. The problem arises when external factors are involved what further destroys human system, which protects us. These factors can be vac_cines (if they contain mercury and viruses or heavy metals from the air, water and food. Heavy metals prevent the binding of vitamin D to the cells, which in the long run causes two negative things: the binding of vitamin D in the cells and the blockade of GMAF. As the long term result, people lose a part of the system, which is responsible for destroying defective cells (including can cells). This part of the body is called T Lym and M1 machages. To put it simply, the system, which protects us from can cells does not function properly anymore. You should know that everybody (healthy as well as sick) has a daily phase of the formation of benign or defective cells, which are produced daily during the division of healthy cells, as a cell defect. Several thousand to several tens of thousands of potential benign and defective cells are generated each day in our bodies as a result of errors in cell division and cell renewal. And these very cells can eventually “go crazy” and turn canous. However, our T Lymphocyte and M1 machages antibodies destruct them on a daily basis. This also explains why some people get can and the others don't. This is because in some people T lymphocytes and M1 machages do not function well (they are poorly active). To those people, whose part of the immune system, which is responsible for destroying defective cells, deteriorates or fails, the daily phase of benign and defective cells slowly multiplies in the place (organ) where the immune system is the weakest. This eventually causes can_cer. However, GMAF can be the solution. IGMAF is the molecule that reactivates, shakes up and gets the T Lymphocytes and M1 machages back on their feet to start destroying benign and damaged cells again, together with potentially already formed canous cells that have accumulated in the body. This system offers a solution for the can treatment, as well as for the prevention of can development. Imunofan 5+ generation helps GMAF in the whole process. Without resetting and activating the SYSTEM (T Lym and M1 Machages) it will be very difficult to beat can_cer in the long term. GMAF helps us to do that. It can be taken as su_ppositories (an_al), capsules or orally. The GMAF protocol lasts approximately for 6 months.
Everyone should be aware of the fact that there are two types of can cells. The first to form at the beginning are "can stem cells" and they are the most resistant to everything and anything. They then later give rise to "daughter can cells", which are less resistant. Oncological therapies are quite successfully performed on daughter can cells, which make majority of can cells or canous masses in the body. However, it is more difficult or it is extremely difficult to destroy stem can cells. This is why, can as a disease “returns” or metas_tasizes
The system “doesn't care”. Without exception (if it is active and functional), it destroys both the stem and daughter can_cer cells. That is why there is so much emphasis on the SYSTEM and its reactivation to begin to recognize can cells again and destroy them.
You may now understand why can often recurs in the long term. Whether the patient is treated with official medic (che_motherapy, radiation,…) or with alternative protocols that destroy can cells, they can only achieve a temporary effect that reduces the canous mass or even destroys it completely. However, after che_motherapy and radiation, the body, which has already been functioning without an active part of the system to destroy can cells (what caused can in the first place), gets another hit as a side effect, which further reduces the functioning of the entire part of the system (it can be seen on the blood work results after che_motherapy). Patients become even more vulnerable and exposed to the formation of can cells. That's why the G_cMAF protocol is so important, after any therapy (medi_cal or alternative). It actually resets and activates human SYSTEM to start guarding and protecting human from getting can again.
MACHAGES DESTROY CAN CELLS.
Once a sufficient quantity of active machages is produced, it is only maintained until the end of the six-month protocol, as machages have a half-life of approximately six days. After 16 to 22 weekly doses of G_cMAF, the amount of Nagse enzymes is expected to fall to a level that is the same as in healthy people, which serves as evidence that the tor has completely been eliminated. The treatment is painless – 100% successful in 16 breast can patients with no recurrence over the next 4 years, as International Journal of Can reports. (International Journal of Can 2008 Jan 15, 122(2):461-7).
Makro_fagi
 Machages (gr. macros - large, phageîn - to swallow) are the largest cells of human system. They carry complete genetic record of a human being and have the capacity to synthesize tens of thousands of different chemicals in order to “fight for” homeostasis and health. In a human body they act as guardians and cleaners, recognizing and neutralizing cells infected with viruses and bacteria, as well as cells with canous changes. They can be found in all tissues. Most of them are found in places of injury or areas with a higher probability of infections (e,g. respiratory tract) etc. At the same time, machages are also an extremely important element in the communication between the innate and the acquired system.
Machages (gr. macros - large, phageîn - to swallow) are the largest cells of human system. They carry complete genetic record of a human being and have the capacity to synthesize tens of thousands of different chemicals in order to “fight for” homeostasis and health. In a human body they act as guardians and cleaners, recognizing and neutralizing cells infected with viruses and bacteria, as well as cells with canous changes. They can be found in all tissues. Most of them are found in places of injury or areas with a higher probability of infections (e,g. respiratory tract) etc. At the same time, machages are also an extremely important element in the communication between the innate and the acquired system.
The majority of machages in human body are in an inactive form and function only within the scope of their regular daily activity. When a critical situation occurs, the GMAF molecule activates them, accelerating their activity by 30 times! To compare with a car: a car can be accelerated from 5 km/h to 150 km/h.
Activation of machages triggers a cascade reaction that activates the entire system.
Nagse enzyme
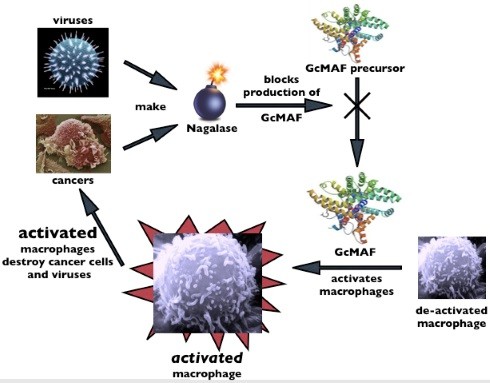 Although human body is extremely “intelligent” viruses and can cells should not be underestimated either! They secrete a special enzyme alpha-N-acetylgalactosaminidase (Naga_lase for short), which blocks the formation of the natural molecule GMAF on the vitamin D axis in the body thus preventing the activation of macro_phages. In this way, the system is literally pushed into a corner and an ideal environment is created, both to grow can cells and the spread of viral diseases at one side, as well as to develop various diseases on the other.
Although human body is extremely “intelligent” viruses and can cells should not be underestimated either! They secrete a special enzyme alpha-N-acetylgalactosaminidase (Naga_lase for short), which blocks the formation of the natural molecule GMAF on the vitamin D axis in the body thus preventing the activation of macro_phages. In this way, the system is literally pushed into a corner and an ideal environment is created, both to grow can cells and the spread of viral diseases at one side, as well as to develop various diseases on the other.
At first, it was thought that the GMAF molecule is only a conductor in the innate part of the system and an exceptional stimulator of macro_phages, and that the therapeutic effect of the GMAF molecule is linked only to the activation of macro_phages that attack and destroy can cells (Nutrints. 2013 Jul 8;5( 7):2577-89). A number of subsequent studies have shown that it has a significantly broader role.
In 2002, the Faculty of Medicine of the University of Nagasaki in Japan published a paper describing the inhibitory effect of the GMAF molecule on angiogenesis (J Natl Can Inst. 2002 Sep 4; 94(17):1211-9). Angiogenesis is the physiological process of the formation of new blood vessels, which supply tor formations with blood and the necessary nutrients for growth and metastasis. GM
In 2010, Lexington University, Kentucky, USA presented the study which showed how GMAF directly inhibits the proliferation and high metastasis potential of can cells in the case of a prostate tor (PLoS One. 2010 Oct 18;5(10):el13428). Less than a year later, the same effect was also demonstrated on breast tor can cells, where it was also shown how it changed the neoplastic phenotype; in other words, can cells treated with GMAF became normal again (Anican Res. 2012 Jan;32(1):45.52).
Based on the aforementioned peer-reviewed scientific articles, we can summarize that the GMAF molecule has several levels of activity that can contribute to successful therapy of can patients:
- Stimulates mahages to attack and destroy can cells,
- Inhibits tor angiogenesis, thereby inhibiting tor growth,
- It directly inhibits the proliferation of can cells and their potential to metastasize and turns can cells back into healthy cells.
The action of the GMAF molecule is complex and significantly broader than just several effects that have been described in this paper. To date, we know of over 20 confirmed effects. Some of them are:
- modulation and reduction of inflammatory mediators,
- reduction of oncogenes,
- increase in the number of natural killer cells,
- increase in neural activity,
- induction of synaptic connection formation,
- increased metabolism in nerve cells,
- reduction of nitrosative stress
- lowering the level of the enzyme nagse,
- reducing the toxic effect of heavy metals.
The GMAF molecule is of utmost importance for the optimal functioning of the system. Comprehensive understanding of its numerous roles is still the subject of many researches. Its already proven effects make it one of the most promising supplements both in general prevention and supportive therapy for many health problems, especially can, cardiovascular diseases, autoimmune diseases and neurodegenerative diseases.
Until recently, the possibility of GMAF molecule therapy was only available to a small circle of people, mainly at private clinics in the form of serum extracts, which represented GMAF generations 1, 2, 3, 4, 5 and 6. After 2010, scientists developed the possibility of developing a bio-identical GMAF molecule from natural ingredients such as cow colostrum and certain micronutrients. Proteins are broken down into peptides during the fermentation process, which, with the help of other micronutrients, form a natural, body-identical GMAF molecule. Today, these preparations are known by the name GMAF 3-6 and the S-6 generation is already on the market. There are few differences between them but the attention should be paid mainly to the quality of the product and the actual absorption, which is guaranteed exclusively by rectal su_ppositories.
Sources (3 different studies):
- Yamamoto N, Naraparaju VR, Moore M, Brent LH: Deglycosylation of serum vitamin D3-binding protein by alpha-N-acetylgalactosaminidase detected in the plasma of patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1997, 82 (3): 290-298. 10.1006/clin.1996.4320.
- Pacini S, Morucci G, Punzi T, Gulisano M, Ruggiero M: Gic pro-derived machage-activating factor (GMAF) stimulates cAMP formation in human mononuclear cells and inhibits angiogenesis in chick embryo chorionallantoic membrane assay. Can Immunol Immunother. 2011, 60 (4): 479-485. 10.1007/s00262-010-0953-7.
- Kanda S, Mochizuki Y, Miyata Y, Kanetake H, Yamamoto N: Effects of vitamin D (3)-binding protein-derived machage activating factor (GMAF) on angiogenesis. J Natl Can Inst. 2002, 94 (17): 1311-1319. 10.1093/jnci/94.17.1311.
Therapies that Reduce Nagse Levels Help to Kill Can Cells
BY ROBERT A.ESLINGER, D.O., H.M.D. | JAN 9, 2020 | HEALTHY MEDICINE
If you read my HB article last month (Dec. 2019) about the can biomarker Nagse, you will recognize its name. If not, what you need to know is that Nagse is an important enzyme produced by can cells and some viruses to help them hide from detection by the immune system. Thus, increased or decreased Nagse levels can be an indicator of the increasing or decreasing presence of can. [For more details, please read the complete article online at HBmag.com, Nagse Test: Newest Can Biomarker.]
Reducing or eliminating Nagse production occurs when can cells are killed. Unfortunately, this usually comes from invasive, toxic chemotherapy and radiation that also harm normal, healthy cells. The good news is, there are options – Nagse-related therapies that do not harm healthy tissue but enable the immune system to identify and eliminate can cells.
These therapies are GMAF and Salicinium, both of which are available in intravenous and oral forms. Their names and modes of action are not familiar to most people, so I will explain.
First, let’s look at GMAF. It is a naturally occurring protein that supports the immune system — a Vitamin D receptor binding protein. The Gc stands for glycoprotein, meaning a protein with one or more sugar molecules attached to it, and the MAF stands for “Machage Activating Factor,” its mechanism of action. Machage white blood cells are part of our immune system. Their main job is to identify and kill any abnormal cells, such as can.
Nagse blocks the Vitamin D receptor sites on the machages so they cannot “see” the can cells. It is an effective survival mechanism for the can.
GMAF therapy works by restoring (activating) the ability of the machages to detect can cells so they can kill them. When this happens, the production of Nagse is reduced and blood levels decrease. It becomes easier for the immune system to detect the presence of any remaining can cells and do its job of destroying them.
Salicinium, the second therapy also lowers Nagse levels. It is a naturally occurring “glyco-benzaldehyde.” The glyco portion is a sugar that can cells use for cellular energy.
Benzaldehyde is substance toxic to abnormal cells like can. Together they are known as a “nano-particle conjugate,” meaning two nano-particles bound together. Nano-particles are extremely small and can be used to carry out certain tasks. They can occur naturally or be chemically engineered.
When Salicinium is delivered to the can cells, sugar is the taxicab and benzaldehyde is the passenger. As the compound enters the can cell, the bonds between the two parts of the conjugate break. The freed benzaldehyde, (toxic only to the energy production pathway in abnormal cells), weakens and/or kills the can. This causes a drop in the Nagse levels that allows the immune system to once again ‘see’ the can cells&
REFERENCES:
- Thyer L, et al. “Gic pro-Derived Machage-Activating Factor Decreases Nagse Levels in Advanced Can Patients,” Oncoimmunology 2013; 2:e25769, Epub 2013 Jul 29.
- Lin CF, et al. “Synthesis and anti-can activity of benzyloxybenzaldehyde derivatives against HL-60 Cells.” Bioorg Med Chem, 2005 Mar 1; 13(5):1537-44.
For further information on both of these therapies contact Reno Integrative Medi_cal Center, 6110 Plumas St., Ste. B, Reno, NV 89519, 775-829-1009, renointegrative.com.
Why don’t we just skip the surgery, radiation and che_motherapy and use GMAF only?
Some can patients, want to avoid surgery, radiation and che_motherapy, and only accept the use of GMAF therapy in their treatment. If GMAF really works, why bother with those annoying procedures at all?
Don't rush your decision. For most can patients, this would be a very bad move. Here's why: GMAF works more effectively on smaller tum_ors. Regardless of the shortcomings of the "big three", they all reduce tumor weight. The basic idea here is that the activated machages "devour" the can cells, but like with lunch - the less you have on your plate, the faster and easier you'll finish your meal. Just as there is an upper limit to the amount of food you can eat, there is also a limit to the size of a tumor that even the most aggressive machages can subdue.
Consider the following: the bigger the dirt, the bigger the sponge you'll need. The same is true with can. Surgery, radiation and che_motherapy reduce the size of the tumor and/or metastases (this is called "debulking"). Smaller tum_ors are an easier target for GMAF-activated machages because they have a smaller number of canous cells and their burden is lower. Patients who dream of a quick, simple and easy treatment should know the following: avoiding the recommended conventional treatments for can treatment is not the smartest move. Using the GMAF method in smaller tum_ors that have already been operated on and in a patient that has already undergone che_motherapy can make the difference between whether the disease is cured or not.
Another mistake is: "I can only try GMAF, and if it doesn't work, I can do surgery, che_motherapy, and/or radiation." This approach is pointless, because waiting can cause the can to grow to a size where there is no turning back. Again, it's best to start with a "debulk" strategy.
Nobuto Yamamoto's research while affiliated with Treatment Research Institute, Philadelphia PA and other places
Retraction Note to: Immun_o_therapy of metastatic colorectal can with vitamin D-binding protein-derived machage-activating factor, GMAF [Can Immunology, Immun_o_therapy,57, (2008) 1007-1016, DOI 10.1007/s00262-007-0431-z]
- Nobuto Yamamoto
- Hirofumi Suyama
- Hiroaki Nakazato
- Yoshihiko Koga
Serum vitamin D binding protein (Gic pro) is the precursor for the principal machage-activating factor (MAF). The MAF precursor activity of serum Gic pro of colorectal can patients was lost or reduced because Gic pro is deglycosylated by serum α-N-acetylgalactosaminidase (Nagse) secreted from canous cells. Deglycosylated Gic pro cannot be converted to MAF, leading to immunosuppression. Stepwise treatment of purified Gic pro with immobilized β-galactosidase and sialidase generated the most potent machage-activating factor (GMAF) ever discovered, but it produces no side effect in humans. Machages treated with GMAF (100 pg/ml) develop an enormous variation of receptors and are highly toricidal to a variety of cans indiscriminately. Administration of 100 nanogram (ng)/human maximally activates systemic machages that can kill canous cells. Since the half-life of the activated machages is approximately 6 days, 100 ng GMAF was administered weekly to eight nonanemic colorectal can patients who had previously received tor-resection but still carried significant amounts of metastatic tor cells. As GMAF therapy progressed, the MAF precursor activities of all patients increased and conversely their serum Nagse activities decreased. Since serum Nagse is proportional to tor burden, serum Nagse activity was used as a prognostic index for time course analysis of GMAF therapy. After 32–50 weekly administrations of 100 ng GMAF, all colorectal can patients exhibited healthy control levels of the serum Nagse activity, indicating eradication of metastatic tor cells. During 7 years after the completion of GMAF therapy, their serum Nagse activity did not increase, indicating no recurrence of can, which was also supported by the annual CT scans of these patients.
Corrigendum to “T.101. Treatment of HIV-infected patients with Gic pro-derived machage activating factor (GMAF) and its coned derivative (GMAFc) eradicates HIV-infection” [Clin. Immunol. 131 (Supplement) (2009) S80]
- Nobuto Yamamoto
- Masumi Ueda
- Kazuya Hashinaka
- Theodore Sery
Abstract 5531: Administration of the most potent machage activating factor, GMAF, to can patients eradicates a variety of can indiscriminately
- Nobuto Yamamoto
- Masumi Ueda
- Kazuya Hashinaka
Proceedings: AACR 102nd Annual Meeting 2011‐‐ Apr 2‐6, 2011; Orlando, FL Intratum_or BCG administration eradicates local as well as metastasized tum_ors (e.g., skin can). Administration of BCG into noncanous tissues, however, results in no effect on the tum_ors. Inflammation induced by BCG in normal tissues releases lysophospholipids that activate machages. Because canous tissues contain alkylphospholipids, BCG-induced inflammation of canous tissues produces alkyl-lysophospholipids and alkylglycerols that activate machages approximately 400 times more effective than lysophospholipids, implying that highly activated machages are tum_oricidal. Inflammation-primed machage activation is the principal machage activation process that requires serum Gic pro (known as vitamin D-binding protein) and participation of B and T lymphocytes. A trisaccharide composed of N-acetylgalactosamine with dibranched galactose and sialic acid termini at 420 threonine residue of Gic pro is hydrolyzed by the β-galactosidase (Bgl) of inflammation-primed B cells and the Neu-1 sialidase of T cells to yield the machage activating factor (MAF), the protein with N-acetylgalactoamine as the remaining sugar. Thus, Gic pro is the precursor for the principal MAF. However, the MAF precursor activity of serum Gic pro of can patients was lost or reduced because Gic pro is deglycosylated by serum α-N-acetylgalactosaminidase (Nagse) secreted from canous cells. Thus, serum Nagse activity is proportional to tum_or burden and serves as an excellent prognostic index. Deglycosylated serum Gic pro can not be converted to MAF, leading to immunosuppression. Stepwise treatment of purified serum Gic pro with immobilized β-galactosidase and sialidase generates probably the most potent MAF (termed GMAF) ever discoverd that produces no side effect in humans. Intramuscular administration of 100 ng GMAF activates systemic machages at maximal level with 100-fold increased ingestion index and 30-fold increased superoxide generating capacity in 6 hrs. These activated machages developed an enormous variation of receptors that recognize cell surface abnormality (known as tum_or associated antigen, TAA) of a variety of can cells and become tum_oricidal to a variety of can indiscriminately. When those can patients bearing breast, prostate, colorectal, stomach, liver, lung, esophagus, kidney, bladder, uterus or ovarian can_cer, or melanoma, fibrosarcoma, leukemia, glioblastoma or mesothelioma were intramuscularly administered with 100 ng GMAF/week, their tum_ors were eradicated in 16 – 54 weeks which is roughly proportional to their original tum_or burden. The curative rate of adenocarcinomas is a biphasic pattern due to a mixture of highly undifferentiated and differentiated tum_or cells while the curative rate of all other can types is linear. In: Proceedings of the 102nd Annual Meeting of the American Association for Can Research; 2011 Apr 2-6; Orlando, FL. Philadelphia (PA): AACR; Can Res 2011;71(8 Suppl):Abstract nr 5531. doi:10.1158/1538-7445.AM2011-5531
Inhibitory effect of vitamin D-binding protein-derived machage activating factor on -induced hamster cheek pouch carcinogenesis and its derived carcinoma cell line
- Yukiyo Toyohara
- Susumu Hashitani
- Hiromitsu Kishimoto
- Kazuma Noguchi
This study investigated the inhibitory effect of vitamin D-binding protein-derived machage-activating factor (GMAF) on carcinogenesis and tum_or growth, using a 9,10-dimethyl-1,2-benzanthracene -induced hamster cheek pouch carcinogenesis model, as well as the cytocidal effect of activated machages against HCPC-1, a cell line established from -induced cheek pouch carcinoma. application induced squamous cell carcinoma in all 15 hamsters of the control group at approximately 10 weeks, and all 15 hamsters died of tum_or burden within 20 weeks. By contrast, 2 out of the 14 hamsters with GMAF administration did not develop tum_ors and the remaining 12 hamsters showed a significant delay of tum_or development for approximately 3.5 weeks. The growth of tum_ors formed was significantly suppressed and none of the hamsters died within the 20 weeks during which they were observed. When GMAF administration was stopped at the 13th week of the experiment in 4 out of the 14 hamsters in the GMAF-treated group, tum_or growth was promoted, but none of the mice died within the 20-week period. On the other hand, when GMAF administration was commenced after the 13th week in 5 out of the 15 hamsters in the control group, tum_or growth was slightly suppressed and all 15 hamsters died of tum_or burden. However, the mean survival time was significantly extended. GMAF treatment activated peritoneal machages in vitro and in vivo, and these activated machages exhibited a marked cytocidal effect on HCPC-1 cells. Furthermore, the cytocidal effect of activated machages was enhanced by the addition of tum_or-bearing hamster serum. These findings indicated that GMAF possesses an inhibitory effect on tum_or development and growth in a -induced hamster cheek pouch carcinogenesis model.
Immun_o_therapy of breast can with Gic pro-derived machage activating factor, GMAF
- Nobuto Yamamoto
- Masumi Ueda
- Kazuya Hashinaka
Proceedings: AACR 102nd Annual Meeting 2011‐‐ Apr 2‐6, 2011; Orlando, FL Serum Gic pro (known as vitamin D-binding protein) is the precursor for the principal machage activating factor (MAF). The MAF precursor activity of serum Gic pro of can patients was lost or reduced because Gic pro was deglycosylated by serum α-N-acetylgalactosaminidase (Nagse) secreted from canous cells. Thus, serum Nagse activity is directly proportional to tumor burden. Machages of can patients having deglycosylated Gic pro cannot be activated. Since machage activation for phagocytosis and antigen-presentation to B cells and T cells is the first indispensable step for development of both humoral and cellular immunities, lack of machage activation leads to immunosuppression. Advanced can patients have high serum Nagse activity, resulting in no machage activation and severe immunosuppression that explain why can patients die with overwhelming infection (e.g., pneumonia). Stepwise treatment of purified Gic pro with immobilized β-galactosidase and sialidase generated probably the most potent machage activating factor (termed GMAF) ever discovered. Because GMAF is structurally identical to the native human MAF, GMAF produces no side effect in humans. Machages activated by GMAF develop an enormous variation of receptors that recognize the abnormality in malignant cell surface and are highly tumoricidal to a variety of cans indiscriminately. When human machages were treated in vitro with GMAF (100 picogram/ml) for 3 hr and a breast can cell line MCF-7 was added with effector/target ratio of 1.5, 60% and 86% of MCF-7 cells were killed in 4 hr and 18 hr incubation, respectively. Administration of 100 nanogram (ng) GMAF per human results in the maximal level of machage activation. Because the activated state of machages is approximately 6 days, sixteen breast can patients received weekly administration of 100 ng GMAF. They regressed in biphasic pattern due to the mixed population of undifferentiated and differentiated cells. After 16-23 weekly administrations of GMAF (100 ng/week), all sixteen patients had very low serum Nagse levels equivalent to those of healthy control values, indicating that these patients are tumor free. No recurrence occurred for more than ten years. GMAF also has a potent mitogenic capacity to act on the myeloid progenitor cells, resulting in a 40-fold increase in systemic machage cell counts in 4 days. Such highly activated systemic machages are chemotactically recruited to inflamed lesions by 180-fold increase of machage cell counts. Since intravenous administration of GMAF allows a rapid interaction of GMAF with myeloid progenitor cells in bone marrow, the systemic cell counts of the activated machages increased to more than 100-fold in 2 days. Weekly intravenous administration of 100 ng GMAF to metastatic breast can patients (n=8) eradicates tumors in 7-15 weeks. In: Proceedings of the 102nd Annual Meeting of the American Association for Can Research; 2011 Apr 2-6; Orlando, FL. Philadelphia (PA): AACR; Can Res 2011;71(8 Suppl):Abstract nr 5532. doi:10.1158/1538-7445.AM2011-5532
Immun_o_therapy of Chronic Lymphocytic Leukemia Patients with Gic pro-derived Machage Activating Factor, GMAF or its Cloned Derivative, GMAFc
- Nobuto Yamamoto
- Masumi Ueda
- Kazuya Hashinaka
Treatment of HIV-Infected Patients with Gic Pro-Derived Machage Activating Factor (GMAF) and Its Coned Derivative (GMAFc) Eradicates HIV-Infection
- Nobuto Yamamoto
- Masumi Ueda
- Kazuya Hashinaka
Immun_o_therapy of Breast and Prostate Can Patients with Gic Pro-Derived Machage Activating Factor, GMAF or Its Cloned Derivative, GMAFc
- Nobuto Yamamoto
- Masumi Ueda
- Kazuya Hashinaka
- Theodore Sery
Treatment of HIV-infected Patients with Vitamin D-binding Protein Derived Machage Activating Factor (GMAF) Eradicates HIV-Infection
- Nobuto Yamamoto
- Kazuya Hashinaka
- Masumi Ueda
- Theodore Sery
Su.69. Immun_o_therapy of Adenocarcinoma Patients with Gic Pro-Derived Machage Activating Factor, GMAF
- Nobuto Yamamoto
- Masumi Ueda
- Kazuya Hashinaka
- Theodore Sery
Immun_o_therapy of metastatic colorectal can with vitamin D-binding protein-derived machage-activating factor, GMAF
- Nobuto Yamamoto
- Hirofumi Suyama
- Hiroaki Nakazato
- Nobuyuki Yamamoto
Serum vitamin D binding protein (Gic pro) is the precursor for the principal machage-activating factor (MAF). The MAF precursor activity of serum Gic pro of colorectal cancer patients was lost or reduced because Gic pro is deglycosylated by serum alpha-N-acetylgalactosaminidase (Nagse) secreted from canous cells. Deglycosylated Gic pro cannot be converted to MAF, leading to immunosuppression. Stepwise treatment of purified Gic pro with immobilized beta-galactosidase and sialidase generated the most potent machage-activating factor (GMAF) ever discovered, but it produces no side effect in humans. Machages treated with GMAF (100 microg/ml) develop an enormous variation of receptors and are highly icidal to a variety of cans indiscriminately. Administration of 100 nanogram (ng)/ human maximally activates systemic machages that can kill canous cells. Since the half-life of the activated machages is approximately 6 days, 100 ng GMAF was administered weekly to eight nonanemic colorectal can patients who had previously received tor-resection but still carried significant amounts of metastatic tor cells. As GMAF therapy progressed, the MAF precursor activities of all patients increased and conversely their serum Nagse activities decreased. Since serum Nagse is proportional to tor burden, serum Nagse activity was used as a prognostic index for time course analysis of GMAF therapy. After 32-50 weekly administrations of 100 ng GMAF, all colorectal cancer patients exhibited healthy control levels of the serum Nagse activity, indicating eradication of metastatic tor cells. During 7 years after the completion of GMAF therapy, their serum Nagse activity did not increase, indicating no recurrence of cancer, which was also supported by the annual CT scans of these patients.
Immun_o_therapy for Prostate Can_cer with Gic Pro-Derived Machage-Activating Factor, GMAF 1
- Nobuto Yamamoto
- Hirofumi Suyama
- Nobuyuki Yamamoto
Serum Gic pro (known as vitamin D(3)-binding protein) is the precursor for the principal macro_phage-activating factor (MAF). The MAF precursor activity of serum Gic pro of prostate can_cer patients was lost or reduced because Gic pro was deglycosylated by serum alpha-N-acetylgalactosaminidase (Naga_lase) secreted from can_cerous cells. Therefore, macro_phages of prostate can_cer patients having deglycosylated Gic pro cannot be activated, leading to immunosuppression. Stepwise treatment of purified Gic pro with immobilized beta-galactosidase and sialidase generated the most potent MAF (termed G_cMAF) ever discovered, which produces no adverse effect in humans. Macro_phages activated by G_cMAF develop a considerable variation of receptors that recognize the abnormality in malignant cell surface and are highly tum_oricidal. Sixteen nonanemic prostate can_cer patients received weekly administration of 100 ng of G_cMAF. As the MAF precursor activity increased, their serum Naga_lase activity decreased. Because serum Naga_lase activity is proportional to tum_or burden, the entire time course analysis for G_cMAF therapy was monitored by measuring the serum Naga_lase activity. After 14 to 25 weekly administrations of G_cMAF (100 ng/week), all 16 patients had very low serum Naga_lase levels equivalent to those of healthy control values, indicating that these patients are tum_or-free. No recurrence occurred for 7 years.
Immun_o_therapy of metastatic breast can patients with vitamin D-binding protein-derived machage activating factor (GMAF) (2008) 122:2 (461-467))
- Nobuto Yamamoto
- Hirofumi Suyama
- Nobuyuki Yamamoto
- Naofumi Ushijima
Serum vitamin D3-binding protein (Gic pro) is the precursor for the principal machage activating factor (MAF). The MAF precursor activity of serum Gic pro of breast can patients was lost or reduced because Gic pro was deglycosylated by serum alpha-N-acetylgalactosaminidase (Nagse) secreted from canous cells. Patient serum Nagse activity is proportional to tor burden. The deglycosylated Gic pro cannot be converted to MAF, resulting in no machage activation and immunosuppression. Stepwise incubation of purified Gic pro with immobilized beta-galactosidase and sialidase generated probably the most potent machage activating factor (termed GMAF) ever discovered, which produces no adverse effect in humans. Machages treated in vitro with GMAF (100 pg/ml) are highly icidal to mammary adenocarcinomas. Efficacy of GMAF for treatment of metastatic breast can was investigated with 16 nonanemic patients who received weekly administration of GMAF (100 ng). As GMAF therapy progresses, the MAF precursor activity of patient Gic pro increased with a concomitant decrease in serum Nagse. Because of proportionality of serum Nagse activity to tor burden, the time course progress of GMAF therapy was assessed by serum Nagse activity as a prognostic index. These patients had the initial Nagse activities ranging from 2.32 to 6.28 nmole/min/mg protein. After about 16-22 administrations (approximately 3.5-5 months) of GMAF, these patients had insignificantly low serum enzyme levels equivalent to healthy control enzyme levels, ranging from 0.38 to 0.63 nmole/min/mg protein, indicating eradication of the tors. This therapeutic procedure resulted in no recurrence for more than 4 years.
Therapeutic Efficacy of the Most Potent Machage Activating Factor (GMAF) for Prostate and Breast Cans
- Nobuto Yamamoto
- Masumi Ueda
- Charles E. Benson
F.128. Machages Activated By GMAF Develop Enormous Variation of Receptors That Recognize and Eradicate Adenocarcinomas
- Nobuto Yamamoto
- Masahiro Urade
- Yoshihiko Koga
- Nobuyuki Yamamoto
Su.59. Fusion (F) Protein Function, α-N-Acetylgalactosaminidase, of Parainfluenza Virus Causes Immunosuppression in Infected Patients
- Nobuto Yamamoto
- Masahiro Urade
- Masumi Ueda
Eradication of HIV by treatment of HIV-infected/AIDS patients with vitamin D-binding protein-derived macrophage activating factor G_cMAF
- Nobuto Yamamoto
- Yoshihiko Koga
- Masumi Ueda
A New Procedure for Experimental Auto IM Uveitis with Small Uveitogenic Peptides
- Theodore W Sery
- Ying-Hsiu Su
- Ralph Eagle
- Masumi Ueda
Demonstration of experimental auto IM uveitis (EAU) with extremely small, fragmented peptides (12-30 amino acid residues) of interphotoreceptor retinoid-binding protein (IRPB). Very small fragmented peptides (no. 854, 888, 907, and 1057) were conjugated to heat-killed Group A Streptococcus cells and administered as a single intravenous injection to Lewis rats. A non-uveitogenic peptide 950 was also conjugated to heat-killed Streptococcus and administered. Administration of a mixture of small peptides and Streptococcus was a control for the peptides conjugated with Streptococcus. The uveitogenic peptide/Streptococcus conjugates produced uveitis inflammatory responses in the uvea, retina and pineal gland. Administration of mixtures of small peptides and Streptococcus cells, and a non-uveitogenic peptide 950 conjugated with Streptococcus did not produce auto IM uveitis. Since mixtures of small uveitogenic peptides and Streptococcal cells did not develop auto IM uveitis, conjugated Streptococcal cells provided a vehicle for machage phagocytosos of very small uveitogenic IRBP peptides. Subsequent antigen presentation from machages to Lym developed auto IM uveitis. Peptide 888, one of four IRBP peptides that encompass the major uveitogenic domain, proved to be the most effective in development of uveitis.
Potent icidal Capacity of Machages Activated by Gic Pro-Derived Machage Activating Factor (GMAF) and Its Therapeutic Efficacy for Prostate, Breast and Colorectal Cans
- Nobuto Yamamoto
- Masahiro Urade
- Masumi Ueda
Pathogenic significance of α-N-acetylgalactosaminidase activity found in the hemagglutinin of influenza virus
- Nobuto Yamamoto
- Masahiro Urade
Serum vitamin D3-binding protein (Gic pro) is the precursor for the principal machage activating factor (MAF). The precursor activity of serum Gic pro was reduced in all influenza virus-infected patients. These patient sera contained alpha-N-acetylgalactosaminidase (Nagse) that deglycosylates Gic pro. Deglycosylated Gic pro cannot be converted to MAF, thus it loses the MAF precursor activity, leading to immun_osuppression. An influenza virus stock contained a large amount of Nagse activity. A sucrose gradient centrifugation analysis of the virus stock showed that the profile of Nagse activity corresponds to that of hemagglutinating activity. When these gradient fractions were treated with 0.01% trypsin for 30 min, the Nagse activity of each fraction increased significantly, suggesting that the Nagse activity resides on an outer envelope protein of the influenza virion and is enhanced by the proteolytic process. After disruption of influenza virions with sodium deoxycholate, fractionation of the envelope proteins with mannose-specific lectin affinity column along with electrophoretic analysis of the Nagse peak fraction revealed that Nagse is the intrinsic component of the hemagglutinin (HA). Cloned HA protein exhibited Nagse activity only if treated with trypsin. Since both fusion capacity and Nagse activity of HA protein are expressed by proteolytic cleavage, Nagse activity appears to be an enzymatic basis for the fusion process. Thus, Nagse plays dual roles in regulating both infectivity and immun_osuppression.
Serum ??-N-Acetylgalactosaminidase Secreted from Canous Cells Serves as Immunodiagnostic and Prognostic Indices for a Variety of Can
- Nobuto Yamamoto
- Masahiro Urade
- Masumi Ueda
Therapeutic Efficacy of Gic Pro-Derived Machage Activating Factor (GMAF) for Adenocarcinoma
- Nobuto Yamamoto
- Masahiro Urade
- Masumi Ueda
Simple method for large-scale production of machage activating factor GMAF
Abstract
Human group-specific component protein (Gic pro) is a multifunctional serum protein which has three common allelic variants, Gc1F, Gc1S and Gc2 in humans. Gc1 contains an O-linked trisaccharide [sialic acid-galactose-N-acetylgalactosamine (GalNAc)] on the threonine420 (Thr420) residue and can be converted to a potent machage activating factor (GMAF) by selective removal of sialic acid and galactose, leaving GalNAc at Thr420. In contrast, Gc2 is not glycosylated. GMAF is considered a promising candidate for immun_otherapy and antiangiogenic therapy of cans and has attracted great interest, but it remains difficult to compare findings among research groups because different procedures have been used to prepare GMAF. Here, we present a simple, practical method to prepare high-quality GMAF by overexpressing Gic pro in a serum-free suspension culture of ExpiCHO-S cells, without the need for a de-glycosylation step. We believe this protocol is suitable for large-scale production of GMAF for functional analysis and clinical testing.
Introduction
Human group-specific component protein (Gc), also known as Gc globulin (GcG) or vitamin D binding protein (DBP), is a 458-amino acid protein with an unmodified mass of 51.2 kDa. Gic pro is mainly synthesized in liver and is present at a high level in blood/plasma (300–600 mg/L), and at lower concentrations in colostrum and milk1,2. It was first described in 19593, and three common allelic variants, Gc1F, Gc1S and Gc2 were subsequently identified in the human population. Relative to the Gc1F protein, Gc1S contains a glutamate residue in place of aspartate at position 416 (D416E mutation), while Gc2 contains a lysine residue in place of threonine at position 420 (T420K mutation)4,5,6. Gic pro has at least four distinct molecular functions. Namely, (1) Gic pro contains a single vitamin D-binding site at N-terminal domain 1, enabling it to work as a carrier protein of vitamin D metabolites in blood2,7,8,9, (2) it has a role in the circulating actin scavenger system, preventing narrowing of small blood vessels by binding to monomeric actin (G-actin) released from damaged cells in the events of cell injury and lysis10,11, (3) it is a precursor of a potent activator of machages (GMAF, Gc-derived machage activating factor) and osteoclasts12,13,14,15, and (4) it serves as a chemotactic cofactor for C5a by interacting with cell-surface proteins of neutrophils16,17.
The C-terminal end of Gc1 (domain III) harbors a single glycosylation site. The carbohydrate structure was firstly elucidated by analysis of the products generated by treatment with several glycolytic enzymes12,18,19, and it was reported that Gc1 contains an O-linked trisaccharide with GalNAc attached to Thr420, followed by a galactose moiety, and a sialic acid (in Gc1F) or mannose moiety (in Gc1S). Gc2, which lacks this threonine residue, was thought to have only a disaccharide moiety composed of GalNAc and galactose, although more than 90% of Gc2 is present as a non-glycosylated form in humans12,18,19. However, current detailed glycan structural analyses using glycosidase treatment and mass spectrometry6,20,21,22 indicated that (1) Gc1F and Gc1S proteins have the same linear trisaccharide, sialic acid-galactose-GalNAc, on the Thr420 residue (Fig. 1 Model B)23 and (2) substitution of a lysine residue at the position corresponding to Thr420 in Gc2 prevents this isoform from being glycosylated at that position, and thus Gc2 is not glycosylated (Fig. 1). These conclusions are supported by the finding that Gc1 from can patients contains the same trisaccharide as Gc1 from healthy volunteers, namely, sialic acid-galactose-GalNAc-Thr42024.
Figure 1
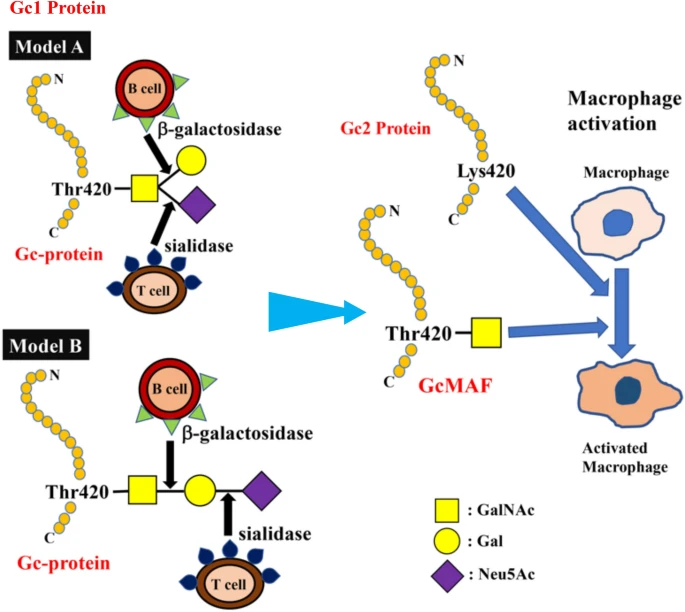
Structure of Gic pro and its conversion to GMAF. Yamamoto et al. proposed (Model A) for the structure of DBP/Gc1F protein and its conversion to GMAF25. In this model, GalNAc is covalently bound to Thr420 of the Gc1F protein and galactose and sialic acid are bound to GalNAc in a Y-branched arrangement. Therefore, removal of galactose and sialic acid exposes the GalNAc moiety and leads to the formation of activated GMAF. However, Ravnsborg et al. recently proposed a linear model (Model B) based on mass spectrometry findings23. In this model, the three sugar moieties attached to threonine 420 are arranged in a linear fashion with GalNAc covalently bound to threonine, and galactose and sialic acid attached to the GalNAc in this order. Non-glycosylated Gc2 is also illustrated based on current mass spectrometry findings.
Gic pros are converted into GMAF via de-glycosylation; specifically, it has been hypothesized that inflammation results in selective hydrolysis of galactose and sialic acid of Gc1 proteins by β-galactosidase of stimulated B lym and sialidase of T lym 13,25,26, leaving GalNAc covalently attached to the threonine residue (Fig. 1). Reported activities of GMAF include machage activation27, anti-angiogenesis activity28,29,30, and anti_tumor activity31,32,33. Indeed, human blood-derived GMAF has been reported to be effective against metastatic colorectal, metastatic breast and prostate cans34,35,36. The medication of GMAF is, therefore, potential options for immun_otherapy and antiangiogenic therapy against cans. However, biological studies and clinical trials of GMAF have yielded inconsistent results, probably because most laboratories and can clinics have produced their own GMAFs using different procedures, even though all are based on sequential de-glycosylation of Gic pros prepared from human blood. Thus, there is a need for methodology to provide a consistent product on a large scale for further studies.
Here, we present a practical method to prepare high-quality GMAF by overexpressing Gic pro in a serum-free suspension culture of ExpiCHO-S cells, without the need for a de-glycosylation step. The synthesized GMAF can be purified by a single step of vitamin D affinity column chromatography. This simple methodology enables the production of large amounts of high-quality GMAF, and should greatly advance functional analyses and further clinical evaluation of GMAF.
Results
Expression of Gc1F in CHO cells
The Gc1F-His expression vector (pcDNA3.4-TOPOGc1F-His) (Supplementary Figs. 1 and 2) was transfected into CHO cells using lipofectamine 2000, and the cells were cultured for 3–4 days. The supernatant was applied to a His Trap HP column, which was washed with binding buffer and eluted with elution buffer solution. The eluate was desalted by dialysis against 50 mM sodium phosphate-buffered saline pH 7.4, and an aliquot of the desalted fraction was de-glycosylated with β-D-galactosidase and sialidase. These preparations were subjected to SDS gel electrophoresis, transferred to a membrane, and stained with anti-human Gc antibody, anti-His antibody and biotin-conjugated Helix promatia (HPA) lectin which roughly reacts to GalNAc. As expected, the expressed protein in the desalted fraction was detected with the antibodies against Gc and His (Fig. 2A), but not with biotin-conjugated HPA lectin (Fig. 2B (−)). However, the protein in the de-glycosylated fraction (Fig. 2B (+))was detected with HPA lectin, suggesting that Gc1F-His protein bearing O-linked trisaccharide (i.e., Sialic acid-Galactose-GalNAc-Thr420)20,21,22,23 was synthesized in CHO cells, and that sialic acid and galactose were removed by the de-glycosylation treatment (Fig. 2B (+)). This result is consistent with reports that Gic pro prepared from human blood can be converted to HPA lectin-reactive GMAF by treatment with β-D-galactosidase and sialidase34,35,36. To examine whether the protein synthesized in CHO cells is N-glycosylated, the desalted His-tagged Gc1F was treated with PNGase and subjected to SDS gel electrophoresis (Fig. 2C). The bands marked by arrowheads showed similar mobilities, suggesting that Gic pro synthesized in CHO cells was not N-glycosylated.
Figure 2
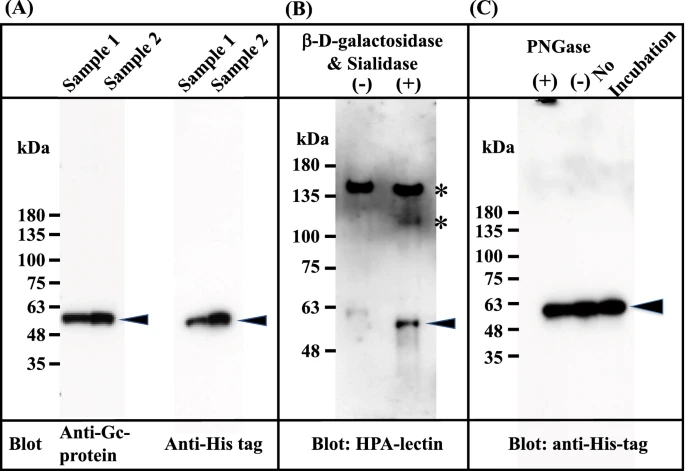
Characterization of Gc1F synthesized in CHO cells. Culture supernatants were adjusted to the composition and pH of the binding buffer of His Trap HP (Ni2+ pre-charged) column chromatography. The samples were applied to the column, which was washed and then eluted with elution buffer. The eluate was desalted by dialysis against 50 mM sodium phosphate pH 7.0 (desalted fraction). The desalted fraction was subjected to 5–20% SDS polyacrylamide gel and bands were blotted onto PVDF membrane. Anti-Gc antibody (A, left panel) and anti-His antibody (A; right panel) were used to detect His-tagged Gc1F and visualized by an enhanced chemiluminescence detection system. Aliquots of desalted His-tagged Gc1F (B (−)) and a sample treated with β-D-galactosidase and sialidase (B (+)) were characterized by HPA-lectin blotting. HPA-lectin-reactive Gc1F is indicated by an arrowhead (B (+)). Extra bands indicated by * are unknown HPA-lectin-reactive protein(s) that were removed during vitamin D affinity column chromatography (see Fig. 4A). N-Glycosylation of Gc-1F synthesized in CHO cells was analyzed (C). Desalted His-tagged Gc1F ((C) no-incubation) was treated with PNGase (C (+)) or incubated without PNGase (C (−)) and subjected to SDS gel electrophoresis. Full-length blots are included in a Supplementary Fig. 4.
Production of HPA lectin-reactive Gic pro without a de-glycosylation step
Based on the above observation, we attempted to establish a standard procedure for large-scale preparation of Gc-protein using serum-free suspension-cultured ExpiCHO-S cells, because this approach has been widely used for large-scale preparation of proteins. Briefly, ExpiCHO-S cells were pre-cultured in serum-free suspension culture medium for 2 days. Expression vector DNA (pcDNA3.4-TOPOGc1F-His) (Supplementary Figs. 1 and 2) was transfected into the pre-cultured cells using Lipofectamine 2000 and culture was continued for 7–8 days; the survival rate of cells gradually decreased and fell below 90% (the recommended criterion to stop culture) at culture days 7 or 8. The culture supernatant was collected by centrifugation at 10,000 × g for 15 min, and applied to a Ni–NTA agarose column. The eluate was desalted by dialysis against phosphate buffer, and then de-glycosylated with β-D-galactosidase and sialidase. The de-salted and de-glycosylated fractions were each characterized by Western blot analysis (Fig. 3).
Figure 3
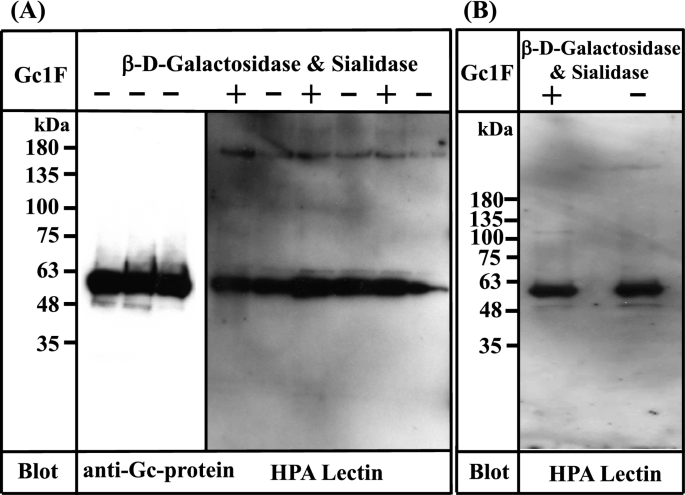
Characterization of Gc1F synthesized in ExpiCHO-S cells and in Expi293-F cells. (A) Three samples of Gc1F-His independently synthesized in ExpiCHO-S cells were characterized using anti-Gc antibody (left panel). Sugar modifications of His-tagged Gc1F alone (−) and after treatment with β-D-galactosidase and sialidase (+) were analyzed by biotin-conjugated HPA-lectin blotting (right panel). (B) Gc1F-His was synthesized in Expi293-F cells under serum-free suspension culture conditions. Sugar modifications of Gc1F-His alone (−) and after treatment with β-D-galactosidase and sialidase (+) were characterized by biotin-conjugated HPA-lectin blotting. Full-length blots are included in a Supplementary Fig. 4.
To our surprise, Gc1F-His expressed in ExpiCHO-S cells was detected not only with anti-Gc antibody (Fig. 3A, left panel), but also with HPA lectin, irrespective of treatment with or without de-glycosylation enzymes (Fig. 3A, right panel). Furthermore, the reactivity to HPA lectin was not further increased by de-glycosylation treatment (Fig. 3A, right panel), suggesting that HPA lectin-reactive saccharide (GalNAc-Thr420) was attached to almost all of the Gc1F-His synthesized in ExpiCHO-S cells under serum free suspension-culture conditions.
We next examined whether the above finding is specific to ExpiCHO-S cells. To test this, we synthesized GcIF-His in Expi293-F cells, another type of cells available for serum-free suspension culture. As shown in Fig. 3B, Gc1F-His expressed in Expi293-F cells was detected with both anti-Gc antibody (data not shown) and biotin-conjugated HPA lectin irrespective of treatment with or without de-glycosylation enzymes, suggesting that HPA lectin-reactive saccharide (GalNAc-Thr420) was present on the expressed protein.
Since three allelic variants, Gc1F, Gc1S and Gc2, are found in humans4,5,6, we next examined whether HPA lectin-reactive forms of Gc1S and Gc2 are also synthesized under serum-free suspension culture conditions, by transfecting pcDNA3.4-TOPOGc1S-His and pcDNA3.4-TOPOGc2-His (Supplementary Fig. 1) into ExpiCHO-S cells. As shown in Fig. 4A, Gc1S-His was detected with biotin-conjugated HPA lectin, irrespective of treatment with or without de-glycosylation enzymes. However, Gc2-His was not detected by HPA lectin even after treatment with de-glycosylation enzymes.
Figure 4
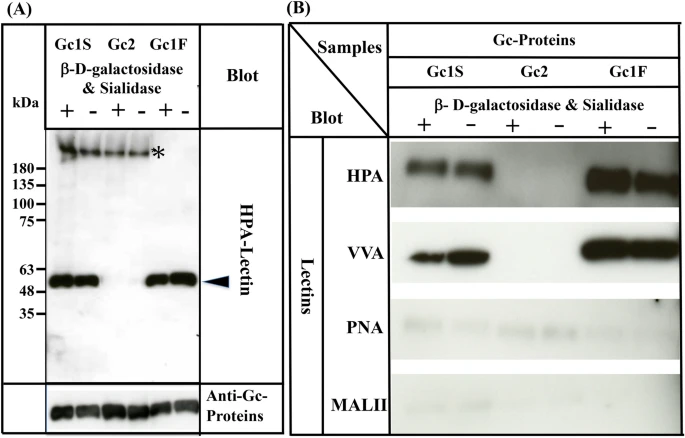
Characterization of Gc1S, Gc2 and Gc1F synthesized by ExpiCHO-S cells in serum-free suspension culture. Gc1S and Gc2 were synthesized by ExpiCHO-S cells transfected with pcDNA3.4-TOPOGc1S-His or pcDNA3.4-TOPOGc2-His (Supplementary Fig. 1) in serum-free suspension culture. Gc1S and Gc2 were purified by His Trap HP (Ni2+ pre-charged) column chromatography and desalted by dialysis against 50 mM sodium phosphate pH 7.0 (desalted fraction). Sugar moieties of the desalted fraction alone (−) and after treatment with β-D-galactosidase and sialidase (+) were analyzed by blotting with biotin-conjugated HPA-lectin (arrowhead). Purified Gc1F was analyzed as a control. Gc1S, Gc2 and Gc1F reacted with anti-Gc antibody (lower column). Extra bands indicated by * were unknown HPA-lectin reactive proteins that became undetectable after vitamin D affinity column chromatography (see lanes 1F+, 1F−). (B) Gc1F, Gc1S and Gc2 synthesized by ExpiCHO-S cells in serum-free suspension culture were purified by His Trap HP (Ni2+ pre-charged) column chromatography and Vitamin D affinity column chromatography, and then desalted by dialysis against 50 mM sodium phosphate pH 7.0 (desalted fraction). Sugar moieties of the desalted fraction without treatment (−) and after treatment with β-D-galactosidase and sialidase (+) were analyzed by blotting with HPA, VVA, PNA, or MALII lectins as indicated. Full-length blots are included in a Supplementary Fig. 4.
Since many lectins only display moderate specificity, we confirmed the above results by using other lectins: biotinylated Vicia villosa (VVA) lectin, biotinylated Maackia amurensis (MAL-II) lectin II and biotinylated peanut (PNA) lectin. As expected, Gc1F and Gc1S reacted strongly with HPA and VVA lectins, which respond to GalNAc/Tn, but scarcely reacted with PNA and MALII lectins, which show a preference for Gal-GalNAc/T disaccharide and sialic acid/STn/ST, respectively (Fig. 4B). The reactions of Gc2 with HPA, VVA, PNA, and MALII lectins were negligible (Fig. 4B). Taken together, these results may indicate that Gc1F and Gc1S produced in ExpiCHO-S cells are glycosylated with GalNAc at Thr420, while Gc2 is not glycosylated.
Purification of Gic pros by vitamin D affinity column chromatography
Gc1 and Gc2 proteins synthesized in ExpiCHO-S cells were purified by His-tag column chromatography and vitamin D affinity column chromatography (Fig. 5A–D). The Gc1 and Gc2 proteins were each purified as a single band of 53,000 Da. We next tested whether the His-tag column chromatography step could be skipped; if so, it should be feasible to produce native Gic pro without the extra tag sequence. To examine this, we synthesized tag-less mouse Gic pro (Supplementary Fig. 1C, pcDNA3.4-TOPOmouse-Gc) in ExpiCHO-S cells and purified the product by a single step of vitamin D affinity column chromatography. The product was detected as a single band of 52,000 Da (Fig. 5E) and confirmed to be HPA lectin-reactive.
Figure 5
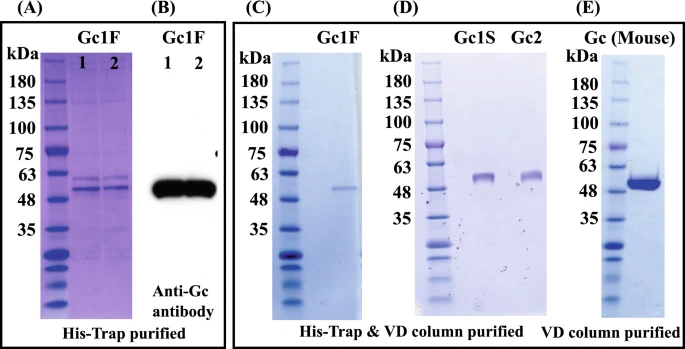
Purification of Gic pros by vitamin D affinity column chromatography. CBB staining patterns of two independently prepared samples of Gc1F, purified with His-Trap column chromatography (A, Gc1F 1 and 2) and blotting patterns with anti-Gc antibody (B, Gc1F 1 and 2). The eluate from the His-Trap column (A, Gc1F 1) was subjected to vitamin D affinity column chromatography. Affinity-purified Gc1F was detected by CBB staining (C). Gc1S and Gc2 were similarly purified and stained with CBB (D). The CBB staining pattern of mouse GMAF purified by a single step of vitamin D affinity column chromatography (E). Full-length gels and blots are included in a Supplementary Fig. 4.
Phagocytosis assay of Gic pros synthesized in ExpiCHO-S cells
We next analyzed the phagocytosis activation activity of Gic pros synthesized in ExpiCHO-S cells. U937 cells were grown in RPMI-1640 medium and differentiated into machage-like cells by means of TPA treatment. Then, U937 cell-derived machages were activated by exposure to purified Gic pros and LPS (a typical machage-activating chemical). Since the phagocytic activity of machages induced by Gc1F derived GMAF is significantly increased at the concentration of 10 ng/ml37, we evaluated the average phagocytosis index (API) of the Gic pros at this concentration. As shown in Fig. 6A, API was increased by the addition of Gic pros and the activation levels of Gc1F and Gc1S were equivalent with that of LPS, used as a positive control, though the activation level of Gc2 was a little lower. We next examined the effect of de-glycosylation by comparing the machage-activating activities of Gc-1F from ExpiCHO-S with a truncated structure (GalNAc-Thr420, Positive control), Gc-1F from CHO with an elongated glycoform (Trisaccharide-Thr420) and Gc-1F from CHO with a truncated glycoform (after de-glycosylation reaction, GalNAc-Thr420). As shown in Fig. 6B, the APIs of Gc-1F from ExpiCHO-S with a truncated structure and Gc-1F from CHO with a truncated glycoform were similarly increased, whereas the activation by Gc-1F from CHO with an elongated glycoform was negligible, suggesting that Gc-1F from CHO is activated by de-glycosylation. We further confirmed that API was dose-dependently increased up to 100 ng/ml for Gc1F, Gc1S and Gc2 (Fig. 6C-1, -2, -3). The actual values obtained in the machage activation assay are given in Supplementary Table 1.
Figure 6
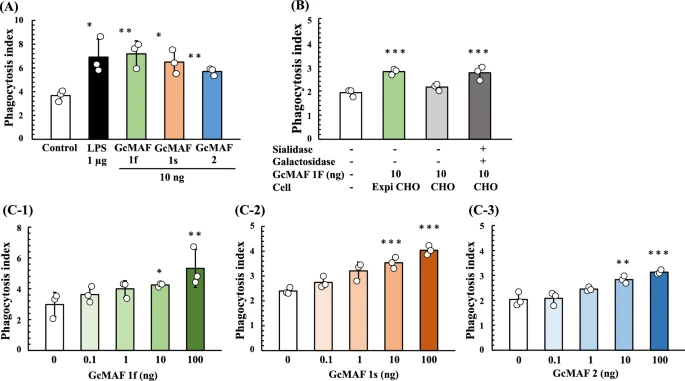
Phagocytosis assay of Gic pros synthesized in ExpiCHO-S cells. Phagocytosis activation activity of vitamin D affinity column-purified GMAF was analyzed. U937 cells were grown in RPMI-1640 medium and differentiated into machage-like cells by means of TPA treatment. (A) Differentiated U937 cells were exposed to Gc1F, Gc1S and Gc2 proteins for 3 h, and then Dynabeads were added to evaluate phagocytotic activity (average phagocytosis index: API). LPS (1 µg) was used as a positive control. (B) APIs of Gc-1F from ExpiCHO-S with a truncated structure (GalNAc-Thr420 type, positive control), Gc-1F from CHO with an elongated glycoform [Trisaccharide-Thr420 type, β-D-galactosidase (−) and Sialidase (−)] and Gc-1F from CHO with a truncated glycoform [GalNAc-Thr420 type, β-D-galactosidase (+) and sialidase (+)]. (C) Dose–response relationships for API of Gc1F (C-1), Gc1S (C-2) and Gc2 (C-3) from ExpiCHO-S. Values are mean ± standard deviation. The statistical significance of differences between control and treatment groups was determined by applying Dunnett's test (*p < 0.05, **p < 0.01).
Discussion
In this work, we present a simple procedure for the large-scale preparation of the phagocytosis-activation active form of Gc, namely GMAF. In humans, the Gc1F and Gc1S allelic variants are synthesized as a phagocytosis-activation inactive and HPA lectin nonreactive forms in the liver, and are converted to phagocytosis-activation active and HPA lectin reactive forms by cleavage of the O-linked sialic acid-galactose-GalNAc-Thr420 to afford the monosaccharide (GalNAc-Thr420). In accordance with this, we found that the phagocytosis-activation inactive and HPA lectin nonreactive form of Gc1F was synthesized in CHO cells and converted to phagocytosis-activation active and HPA-lectin reactive form by the action of de-glycosylation enzymes (β-D-galactosidase and sialidase) (Figs. 2B, 6C). However, to our surprise, phagocytosis-activation active and HPA lectin reactive Gc1F was directly synthesized in ExpiCHO-S cells and in Expi293-F cells under serum-free suspension culture conditions. Furthermore, Gc1S and Gc2 were also synthesized as phagocytosis-activation active forms in ExpiCHO-S cells (Fig. 6). As expected, Gc1F and Gc1S were reactive with HPA and VVA-lectins. However, Gc2 (T420K mutation) was not. This suggests that (1) HPA-lectin-reactive saccharide may be not essential for the phagocytosis-activation activity of Gc2 protein, at least in vitro, and (2) if so, Gc2 synthesized in ExpiCHO-S cells may act as a phagocytosis-activation factor via a mechanism not involving GalNAc. This may support the hypothesis that the presence or absence of GalNAc at Thr420 of Gic pro is irrelevant in determining immune competency and/or can risk38, and may also be consistent with the fact that the risk of can in Gc2-2 homozygotes is decreased rather than increased, even though they are unable to produce GMAF containing GalNAc-Thr42039.
A stereo view of the overall fold structure of human Gic pro 40. indicates that amino acids 418–420, including the glycosylation site, are located in the vicinity of the loop-helix (H3) structure, and the sugar moiety seems to be located on the exterior of a globular conformation of H3 (Supplementary Fig. 3)41. Thus, the truncated sugar moiety (GalNAc-Thr420) could be recognized by a putative receptor protein on the cell surface of machages and might initiate complex formation between Gc1 protein and the putative receptor (Supplementary Fig. 3B). However, the recognition/interaction between the sugar moiety and putative receptor alone might be not sufficient for functional/stable complex formation and protein–protein interaction between Gc1 protein and the putative receptor would be required for the formation of a functional/stable complex to transduce the machage activation signal (Supplementary Fig. 3B). Thus, in this system, GalNAc-Thr420 may function as a marker for molecular recognition by the putative receptor, triggering the protein–protein interaction. In contrast, the trisaccharide attached to Thr420 is not recognized by the putative receptor and thus cannot initiate protein–protein interaction. Furthermore, the trisaccharide attached to Thr420 may interfere with the interaction between Gc1 protein and the putative receptor (Supplementary Fig. 3A). This might be the reason why the truncation of sialic acid and galactose is required for the conversion of inactive Gc protein to active Gc_MAF. Since Gc2 has no sugar chain, such interference with protein–protein interaction between Gc2 and the putative receptor protein would not occur (Supplementary Fig. 3C). Although it is still unclear how the putative receptor can recognize Gc2 without GalNAc on Thr420, the amino acid substitution (T420K mutation) in Gc2 might favor interaction with the putative receptor and if so, Gc2 itself might be always functional, without activation. Such a non-regulated interaction between Gc2 and the putative receptor may explain why the risk of can in Gc2-2 homozygotes is decreased rather than increased39. Thus, our results provide an intriguing clue to the possible role(s) of the glycan moiety in machage activation signaling.
Our present data indicate that Gc1 protein synthesized in ExpiCHO-S cells is almost entirely HPA-lectin-reactive, suggesting that the newly synthesized Gc1 bears only the monosaccharide (GalNAc-Thr420), and the following extension reactions of the O-glycan do not occur. Although the underlying mechanism of production of this truncated O-glycan is unknown, it seems likely that the step-by-step glycosylation process in the Golgi apparatus is altered in ExpiCHO-S cells. Several tor cell lines show altered distribution or partial loss of O-glycosylation enzymes in the Golgi apparatus42, and alteration of the O-glycan biosynthesis pathway(s) might occur similarly in ExpiCHO cells. Alternatively, Gc1 protein might undergo premature exit from an early compartment of the Golgi apparatus. Thus, our results provide an interesting future direction for further studies of the machinery of protein glycosylation and secretion.
In conclusion, we established methodology to produce biologically active GMAF in large quantities by overexpressing Gic pros in ExpiCHO-S cells under serum-free suspension culture conditions. Notably, the synthesized GMAF can be purified in a single step by means of vitamin D affinity column chromatography. This simple protocol is expected to be suitable for large-scale production of high-quality GMAF for functional analysis and clinical testing.
Materials and methods
Construction of Gc-protein expression vectors
We obtained a double-stranded DNA encoding a fusion protein consisting of human Gc1F protein and a histidine tag (His) at the C-terminal site (Gc1F-His DNA) by using the Oligo-DNA production service of Invitrogen (Supplementary Fig. 1A). Some triplet codons were modified to accord with the codon usage rates in animal cells in order to maximize the efficiency of translation. Gc1F-His DNA was inserted into the TOPO cloning site of pcDNA3.4-TOPO Vector (Invitrogen) (termed pcDNA3.4-TOPOGc1F-His), in which the inserted Gc1F-His DNA is transcribed under the control of CMV promoter (Supplementary Fig. 2). To design Gc1S-His DNA, the GAT codon for aspartic acid416 (D416) was replaced with the GAG codon for glutamic acid (E416) (termed pcDNA3.4-TOPOGc1S-His, Supplementary Fig. 1B-1). To design Gc2-His DNA, the ACC codon for threonine420 (T420) was replaced with the AAG codon for lysine (K420) (termed pcDNA 3.4-TOPOGc2-His) (Supplementary Fig. 1B-2)4,5,6. We also synthesized a mouse Gc expression plasmid (pcDNA3.4-TOPOmouse-Gc) (Supplementary Fig. 1C). Large amounts of these expression vector DNAs were prepared by using an EndoFree Plasmid Kit (QIAGEN 12362) according to the supplier’s protocol.
CHO cell culture and transfection of Gc1F-His expression plasmid
Chinese hamster ovary cells (CHO) were cultured in F12 medium (GIBCO 11765) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Biowest S1820), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich P4333) at 37 °C in a humidified atmosphere of 5% CO2. Cells were cultured to approximately 80% confluence in 6-well culture plates. The pcDNA3.4-TOPOGc1F-His expression plasmid (2.5 μg) and Lipofectamine 2000 (15 μl) (ThermoFisher Scientific Co.) were added in 2 ml medium per well. The plates were incubated for 3 days, then the culture media and cells were harvested. The expression level and sugar modification of Gc1F-His were evaluated.
Culture of ExpiCHO-S and Expi293-F cells
ExpiCHO-S cells (ThermoFisher Scientific Co. ExpiCHO-S Cells, Catalog number: A29127) were suspension-cultured in 25 ml of serum-free ExpiCHO Expression medium in an incubator at 37 °C with a humidified atmosphere of 8% CO2 in air on an orbital shaker platform rotating at 125 rpm to a final density of 6 × 106 viable cells/ml. A Gc expression plasmid (15 μg) (pcDNA3.4-TOPOGc1F-His, pcDNA3.4-TOPOGc1S-His, pcDNA3.4-TOPOGc2-His or pcDNA3.4-TOPOmouse Gc) was diluted with 1.0 ml of Opti PRO SFM (ThermoFisher Scientific Co.) and mixed with 80 μl of ExpiFectamine CHO Reagent (ThermoFisher Scientific Co.), previously diluted with 920 μl of Opti PRO SFM. This mixture was incubated for 5 min at room temperature and added to the ExpiCHO-S cell culture flask. The cells were incubated overnight, then 150 μl of ExpiCHO Enhancer and 6 ml of ExpiCHO Feed (ThermoFisher Scientific Co.) were added, and suspension culture was continued for 6–8 days (according to the supplier’s protocol, cell culture can be continued for up to 14 or 15 days). Then, we harvested the cells and media and analyzed the expression levels and sugar modifications of the Gc proteins.
Expi293F cells (ThermoFisher Scientific Co. Expi293F cells, Catalog number: A14528) were suspension-cultured in 30 ml of Expi293 Expression medium in an incubator at 37 °C with a humidified atmosphere of 8% CO2 in air on an orbital shaker platform rotating at 125 rpm to a level of 2.5 × 106 cells/ml with > 95% viability. Gc expression plasmids (30 μg) were diluted with 1.5 ml of Opti-MEM (ThermoFisher Scientific Co.) and mixed with 80 μl of Expifectamine 293 reagent (ThermoFisher Scientific Co.), previously diluted with Opti-MEM to 1.5 ml. This mixture was incubated for 20 min at room temperature and then added to an Expi293-F cell culture flask. The cells were incubated under the conditions specified above and, after 18 h, 150 μl of Expifectamine 293 Transfection Enhancer 1 and 1.5 ml of Expifectamine 293 Transfection Enhancer 2 (ThermoFisher Scientific Co.) were added to the flask. The cells and the media were harvested at 3 days post-transfection. The expression levels and sugar modifications of the produced Gic pros were characterized.
Purification of Gc-His proteins with a His-Tag column
For the purification of histidine-tagged Gic pros, we performed His Trap HP (Ni2+ pre-charged) column chromatography (GE Healthcare Life Sciences) according to the manufacturer’s protocol. Briefly, (1) the His Trap HP column (5 ml) was washed with binding buffer (20 mM sodium phosphate, 150 mM NaCl and 20 mM imidazole pH 7.4), (2) the cell culture supernatant was adjusted to the composition and pH of the binding buffer and filtered through a 0.45 μm filter, and (3) the filtrate was applied to the column, which was washed with binding buffer and eluted with elution buffer (20 mM sodium phosphate, 150 mM NaCl and 500 mM imidazole pH 7.4). Desalting was done by dialysis against 50 mM sodium phosphate pH 7.0.
Assessment of cell proliferation and cell numbers
We determined the viable and total cell counts by means of the trypan-blue exclusion method. Cell suspensions were diluted with 0.5% trypan-blue solution and the cells were counted on a Neubauer hematocytometer.
Quantitation of total protein
Protein concentrations were determined with BCA Protein Assay Kit (Thermo Fisher 23227) or by measuring the absorbance at 280 nm.

Western blot analysis of Gic pros and lectin blot for glycan moieties
The synthesized human Gc-His proteins were mixed with equal amounts of SDS sampling buffer (125 mM Tris–HCl pH 6.8, 4% SDS, 60% glycerol, 10 mM EDTA, 0.1 M DTT, 0.01% BPB, 10% 2-mercaptoethanol) and heated at 90 °C for 2 min. Samples were electrophoresed on 5–20% SDS polyacrylamide gel (Nacalai tesque, Kyoto, Japan) and bands were transferred to PVDF membranes (Merck Millipore Ltd., ME) using a semi-dry blotting device. The blot was incubated at 4 °C overnight with anti-Gc antibody (Abcam, ab153922) or with anti-His-tag antibody (Abcam ab18184), previously diluted in a blocking buffer containing 5% skim milk in TBST (25 mM Tris–HCl pH 7.4, 150 mM NaCl and 0.1% Tween 20). The primary antibodies, anti-DBP/Gc and anti-His-tag antibodies, were reacted with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (NA934, GE Healthcare) and anti-mouse IgG (GE Healthcare, NA9310v), respectively, and visualized by use of an enhanced chemiluminescence detection system (ECL prime, Amersham Bioscience). Biotin-conjugated Helix pomatia (HPA) lectin for Tn (Sigma-Aldrich, L6512) was used to detect the GalNAc/Tn moiety as previously reported37. In addition, biotin-conjugated Vicia villosa (VVA) lectin for GalNAc/Tn (Vector Laboratories, B-1235), biotin-conjugated Maackia amurensis lectin II (MAL-II) for sialic acid/ STn/ST (Vector Laboratories, B-1265) and biotin-conjugated peanut (PNA) lectin for Gal-GalNAc/T disaccharide (GeneTex, BTX01507) were used to confirm the glycosylation of Gc1 and Gc2 proteins.
Purification of Gic pros by vitamin D-sepharose column chromatography
The suspension-culture supernatant or His-trap column eluate was applied to a 25(OH)D3-sepharose column prepared according to Link et al.43. The column was washed with binding buffer (50 mM Tris–HCl, 1.5 mM EDTA, 150 mM NaCl and 0.1% Triton X-100 pH 7.4) and eluted with 6 M guanidine. For desalting, the eluate was dialyzed against 10 mM sodium phosphate pH 7.4.
Phagocytosis assay
The phagocytosis activity of GMAF was assayed according to the reported method44 with slight modifications. U937 cells (human myeloid leukemia cell line; KAC Co., Ltd., Ritto, Japan) were grown in RPMI-1640 medium (Gibco by Life Technologies, Grand Island, USA) supplemented with 10% (v/v) heat-inactivated FBS (Gibco Life Technologies, Grand Island, USA) and penicillin–streptomycin (Sigma-Aldrich, USA) in a fully humidified 5% CO2/95% air atmosphere at 37 °C. To induce differentiation into machage-like cells, 2.5 × 106 U937 cells seeded onto 10 cm culture dishes were treated with 10 ng/ml 12-O-tetra-decanoylphorbol-13-acetate (TPA) (Nacalai tesque Co., Ltd., Kyoto, Japan) for 3 days, washed with 3 ml of Dulbecco’s PBS (Gibco Life Technologies Grand Island, USA) twice, and then trypsinized (1 ml of trypsin–EDTA per 10 cm dish) for 2–3 min at 37 °C. Then 2.5 × 105 trypsinized cells were dispersed in 10% FBS/RPMI-1640 medium, layered onto cover-glasses in a 24-well plate, and treated with 10 ng/ml TPA for 3 days. These cells were incubated with serum-free RPMI-1640 medium for 15 h and pretreated with GMAF for 3 h by replacing the medium with serum-free RPMI-1640 medium containing 10 ng/ml GMAF1f, GMAF1s, or GMAF2. LPS (1 µg/ml) was used as a positive control. After 3 h pretreatment, the medium was replaced by serum-free RPMI-1640 medium containing 90 μg/ml protein G magnetic beads (Dynabeads Protein G; Invitrogen, Carlsbad, USA) and incubated for 1.5 h. The cover-glasses were washed with phosphate-buffered saline and the cells were fixed with methanol for 1 min. The fixed cells were air-dried, stained with Giemsa’s azur eosin methylene blue solution (Merck KGaA, Darmstadt, Germany) for 1 h and washed with distilled water. The cells were air-dried, and then immersed in Malinol (Muto Pure Chemicals Co., Ltd., Tokyo). Cells were imaged with an upright microscope (DM6B, Leica Microsystems, Germany); nine random areas in each cover-glass sample were examined. The activity of GMAF was expressed as the average phagocytosis index (API), calculated according to the following formula: API = (number of internalized beads within the machages)/(number of machages within the photograph). We prepared three independent samples for each analysis and thus 27 areas (9 random areas × 3 independent samples) were analyzed for each sample. The above examination was repeated twice.

Statistical analysis
All values are expressed as mean ± standard deviation. Statistical analyses were performed with EZR (Saitama Medi_cal Center, Jichi Medi_cal University, Saitama, Japan)45, which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria); specifically, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics. The statistical significance of differences between control and treatment groups was determined by use of Dunnett’s test.
References
- Daiger, S. P. Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D. Proc. Natl. Acad. Sci. USA 72, 2076–2080 (1975).
- Chun, R. F. New perspectives on the vitamin D binding protein. Cell Biochem. Funct. 30, 445–456 (2012).
- Thomas, W. C. et al. Studies on the anti-ricketic activity in sera from patients with disorders of calcium metabolism and preliminary observations on the mode of in human serum. J. Clin. Invest. 38, 1078–1085 (1959).
- Cleve, H. & Constans, J. The mutants of the vitamin-D-binding protein: more than 120 variants of the GC/DBP system. Vox. Sang. 54, 215–225 (1988).
- Speeckaert, M., Huang, G., Delanghe, J. R. & Taes, Y. E. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin. Chim. Acta 372(1–2), 33–42 (2006).
- Borges, C. R. et al. Full-length characterization of proteins in human populations. Clin. Chem. 56, 202–211 (2010).
- Haddad, J. G. et al. Identification of the sterol- and actin-binding domains of plasma vitamin D binding protein (Gc-globulin). Biochemistry 31, 7174–7181 (1992).
- Addo, J. K., Swamy, N. & Ray, R. The C(19) position of 25-hydroxyvitamin D(3) faces outward in the vitamin D sterol-binding pocket of vitamin D-binding protein. Bioorg. Med. Chem. Lett. 12, 279–281 (2002).
- Verboven, C. et al. A structural basis for the unique binding features of the human vitamin D-binding protein. Nat. Struct. Biol. 2, 131–136 (2002).
- Lind, S. E., Smith, D. B., Janmey, P. A. & Stossel, T. P. Role of plasma gelsolin and the vitamin D-binding protein in clearing actin from the circulation. J. Clin. InVest. 78, 736–742 (1986).
- Lee, W. M. & Galbraith, R. M. The extracellular actin-scavenger system and actin toxicity. N. Engl. J. Med. 326, 1335–1341 (1992).
- Yamamoto, N. & Homma, S. Vitamin D3 binding protein (group-specific component) is a precursor for the machage activating signal factor from lysophosphatidylcholine-treated Lym. Proc. Natl. Acad. Sci. USA 88, 8539–8543 (1991).
- Yamamoto, N., Lindsay, D. D., Naraparaju, V. R., Ireland, R. A. & Popoff, S. N. A defect in the inflammation-primed machage-activation cascade in osteopetrotic rats. J. Immunol. 152, 5100–5107 (1994).
- Schneider, G. B., Benis, K. A., Flay, N. W., Ireland, R. A. & Popoff, S. N. Effects of vitamin D binding protein-machage activating factor (DBP-MAF) infusion on bone resorption in two osteopetrotic mutations. Bone 16, 657–662 (1995).
- Swamy, N., Ghosh, S., Schneider, G. B. & Ray, R. Baculovirus-expressed vitamin D-binding protein-machage activating factor (DBP-maf) activates osteoclasts and binding of 25-hydroxyvitamin D3 does not influence this activity. J. Cell. Biochem. 81, 535–546 (2001).
- DiMartino, S. J. & Kew, R. R. Initial characterization of the vitamin D binding protein (Gc-globulin) binding site on the neutrophil plasma membrane: Evidence for a chondroitin sulfate proteoglycan. J. Immunol. 163, 2135–2142 (1999).
- McVoy, L. A. & Kew, R. R. CD44 and annexin A2 mediate the C5a chemotactic cofactor function of the vitamin D binding protein. J. Immunol. 175, 4754–4760 (2005).
- Svasti, J., Kurosky, A., Bcnnett, A. & Bowman, B. H. Molecular basis for the three major forms of human serum vitamin D binding protein (group specific component). Biochemistry 18, 1611–1617 (1979).
- Yamamoto, N. Structural definition of a potent mahage activating factor derived from vitamin D3-binding protein with adjuvant activity for antibody production. Mol. lmmunol. 33, 1157–1164 (1996).
- Borges, C. R., Jarvis, J. W., Oran, P. E. & Nelson, R. W. Population studies of Vitamin D Binding Protein microheterogeneity by mass spectrometry lead to characterization of its genotype-dependent O-glycosylation patterns. J. Proteome Res. 7, 4143–4153 (2008).
- Borges, C. R. & Rehder, D. S. Glycan structure of Gic Pro-derived macage activating factor as revealed by mass spectrometry. Arch. Biochem. Biophys. 606, 167–179 (2016).
- Kilpatrick, L. E. & Kilpatrick, E. L. Optimizing high-resolution mass spectrometry for the identification of low-abundance post-translation modifications of intact proteins. J. Proteome Res. 16, 3255–3265 (2017).
- Ravnsborg, T. et al. The glycosylation and characterization of the candidate Gc machage activating factor. Biochim. Biophys. Acta 1804, 909–917 (2010).
- Rehder, D. S., Nelson, R. W. & Borges, C. R. Glycosylation status of vitamin D binding protein in can patients. Protein Sci. 18, 2036–2042 (2009).
- Yamamoto, N. & Kumashiro, R. Conversion of vitamin D3 binding protein (group-specific component) to a machage-activating factor by the stepwise action of beta-galactosidase of B-cells and sialidase of T-cells. J. Immunol. 151, 2794–2802 (1993).
- Mohamad, S. B., Nagasawa, H., Uto, Y. & Hori, H. Preparation of Gic pro-derived machage activating factor (GMAF) and its structural characterization and biological activities. Anticanr Res. 22, 4297–4300 (2002).
- Nagasawa, H., Sasaki, H., Uto, Y., Kubo, S. & Hori, H. Association of the machage activating factor (MAF) precursor activity with polymorphism in vitamin D-binding protein. Antican Res. 24, 3361–3366 (2004).
- Kanda, S., Mochizuki, Y., Miyata, Y., Kanetake, H. & Yamamoto, N. Effects of vitamin D3-binding protein-derived machage activating factor (GMAF) on angiogenesis. J. Natl. Can Inst. 94, 1311–1319 (2002).
- Kisker, O. et al. Vitamin D binding protein-machage-activating factor (DBP-maf) inhibits angiogenesis and tor growth in mice. Neoplasia 5, 32–40 (2003).
- Pacini, S., Morucci, G., Punzi, T., Gulisano, M. & Ruggiero, M. Gic pro-derived machage-activating factor (GMAF) stimulates cAMP formation in human mononuclear cells and inhibits angiogenesis in chick embryo chorionallantoic membrane assay. Can Immunol. Immunother. 60, 479–485 (2010).
- Koga, Y., Naraparaju, V. R. & Yamamoto, N. Anti effect of vitamin D-binding protein-derived machage-activating factor on Ehrlich ascites tor-bearing mice. Proc. Soc. Exp. Biol. Med. 220, 20–26 (1999).
- Mohamad, S. B. et al. Gic pro-derived machage activating factor (GMAF): Isoelectric focusing pattern and icidal activity. Antican Res. 23, 4451–4457 (2003).
- Nonaka, K. Vitamin D binding protein-machage activating factor inhibits HCC in SCID mice. J. Surg. Res. 172, 116–122 (2012).
- Yamamoto, N., Suyama, H. & Yamamoto, N. Immun_o_therapy for prostate can with Gic pro-derived machage-activating factor. GMAF. Transl. Oncol. 1, 65–72 (2008).
- Mohamad, S. B., Hori, H., Nagasawa, H., Usui, K. & Uto, Y. Characterization of human Gic pro-derived machage activation factor (GMAF) and its functional role in machage icidal activity. Adv. Exp. Med. Biol. 510, 77–82 (2003).
- Gregory, K. J. et al. Vitamin D binding protein-machage activating factor directly inhibits proliferation, migration, and uPAR expression of prostate can cells. PLoS ONE 5, e13428 (2010).
- Uto, Y. et al. Effect of the Gc-derived machage-activating factor precursor (PreGMAF) on phagocytic activation of mouse peritoneal machages. Antican Res. 31, 2489–2492 (2011).
- Ruggiero, M., Reinwald, H. & Pacini, S. Is chondroitin sulfate responsible for the biological effects attributed to the GiC pro-derived Machage Activating Factor (GMaf)?. Med. Hypotheses 94, 126–131 (2016).
- Abbas, S. et al. The Gc2 allele of the vitamin D binding protein is associated with a decreased postmenopausal breast can risk, independent of the vitamin D status. Can Epidemiol. Biomarkers Prev. 17, 1339–1343 (2008).
Berman, H. M. et al. The Protein Data Bank. Acta Crystallogr. D Biol. Crystallogr. 58, 899–907 (2002).
- Verbovcn, C. et al. A structural basis for the unique binding features of the human vitamin D-binding protein. Nat. Struct. Bio. 19, 131–136 (2002).
- Brockhausen, I. Mucin-type O-glycans in human colon and breast can: glycoldynamics and functions. EMBO Rep. 7, 599–604 (2006).
- Link, R. P., Perlman, K. L., Pierce, E. A., Schnoes, H. K. & DeLuca, H. F. Purification of human serum vitamin D-binding protein by 25-hydroxyvitamin D3-sepharose chromatography. Anal. Biochem. 157, 262–269 (1986).
- Ishikawa, M. et al. A novel assay system for machage-activating factor activity using a human U937 cell line. Antican Res. 34, 4577–4582 (2014).
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medi_cal statistics. Bone Marrow Transpl. 48, 452–458 (2013).
GMAF: our next-generation immun_o_therapy
Saisei Mirai, based in Osaka, Japan, is a medi_cal organization specializing in can immun_o_therapy. The name Saisei Mirai is derived from the Japanese words for regeneration and future. It is our mission to provide can patients with the latest, most effective treatments while also ensuring the best possible quality of life. Can immun_o_therapy is the use of the IM system to reject can. In particular, it involves stimulating the patient’s IM system to locate and eliminate the cells that are canous. This is achieved with IM cells such as Natural Killer cells (NK cells), T Lymphocytes, Dendritic Cells (DC), and machages, among others. A variety of treatments, such as GMAF, Hyper T/NK cells, Coley vac_cine, gene therapy, can vac_cines, and intravenous high-dose vitamin C or α-lipoic acid are routinely used to stimulate the IM system.
The IM system is the body’s vital self defense that protects against disease. It can recognize a wide variety of pathogens found in the environment and even tum_or cells borne within the human body. The mechanisms of protection fall into two broad categories — innate immunity and adaptive immunity. There are several types of cells in the innate IM system: phagocytic neutrophils; machages; dendritic cells; mast cells; and natural killer (NK) cells. The adaptive IM system is comprised of the lymphocytes, both T cells and B cells, which are able to recognize and remember specific pathogens, and their products, including antibodies.
The progression of many diseases, such as can and AIDS, follows the collapse of the IM system. We now know that stimulating the IM system can halt or even reverse such diseases. Immun_o_therapy has become an attractive new strategy in the treatment of can1. The laboratory and clinical study of can immun_o_therapy — such as dendritic cells, autologous lymphocyte-activated killer (LAK) cells, autologous NK cells, monoclonal antibodies, can peptide vac_cines, cytokines, and biological response modifiers (BRM) — is rapidly advancing. However in a clinical setting, the results of can immun_o_therapy are mixed.
We will review first-generation GMAF developed by Dr Yamamoto and secondgeneration GMAF currently under development at Saisei Mirai (Fig. 1). We will also present a number of other therapies used in combination with GMAF.
At Saisei Mirai, we are currently treating can patients with GMAF-based can immun_o_therapy. We use GMAF in combination with other IM system-related therapies, such as regional and whole-body hyperthermia, Coley vaccine, which stimulates fever within the body, and Hyper-T/ NK-cell therapy. All of these therapies are about strengthening the IM system and take a holistic approach to fighting can rather than a localized approach that is common with conventional therapies such as radiation and surgery.
Coley vac_cine
Coley vac_cine — also known as Coley’s Toxins and Coley Fluid — was one of the first immun_o_therapies to enter the clinic. Coley-vaccine therapy is a medi_cal treatment used to induce a fever to improve the immunological competence of the patient. For hundreds of years, doctors have reported cases of tors that disappeared, apparently spontaneously after patients developed high fevers from infection. In many cases, when fever subsided, tors had broken down and been absorbed or sloughed off. Infection seemed to be key to these miraculous cures. It was not until 1893 when Dr William Coley began injecting a sterile mixture of Streptococcus pyogenes and Serratia marcescens bacteria into can patients to induce fevers, that this observation was put into clinical practice. By using a suspension of killed bacteria, Coley was able to mimic an acute infection, without the risks of an actual pathogen.
Hyperthermia
Hyperthermia heat therapy, also called thermotherapy, is the application of heat to the body in order to treat a medi_cal condition (Fig 2). It is used to activate a patient’s immunological competence and is often utilized in combination with other medi_cal treatments at Saisei Mirai.
At Saisei Mirai a patient can select from a wide range of suitable and compassionate integrative medi_cal treatments in consultation with doctors experienced with can immun_o_therapy. Recently, immun_o_therapy treatments are being administered to people living with with infections, such as HIV, hepatitis B virus (HBV) and hepatitis C virus (HCV).
Saisei Mirai has a rich history in can research. It has collaborated with a number of renowned research centers such as Tokushima University, Kagoshima University and Kobe University and has contributed significantly to the clinical application of new can therapies.
Discovery and basic study of fi rstgeneration GMAF
GMAF, discovered by Dr Nobuto Yamamoto in 1991, is derived from the group specific component (Gic) pro (vitamin D binding protein), a member of the albumin superfamily2. GMAF is an interesting serum glycoprotein with various biological activities. It is reported that during an inflammatory response, β-galactosidase of an activated B-cell and sialidase of a T-cell hydrolyze the terminal galactose and sialic acid saccharides of Gic pro to produce GMAF3. GMAF has interesting biological activity; it activates machages via superoxide radical generation and phagocytic activation4, and has been demonstrated to have antiangiogenic and anti-tor activity in vivo5. GMAF also directly inhibits proliferation and migration of human prostate can cells or human breast can cells independent of its machage activation ability6.
Figure 1: Research map of Gic pro and GMAF
First-generation GMAF developed by Dr Nobuto Yamamoto. Second-generation GMAF proposed by Dr Yoshihiro Uto, Dr. Hitoshi Hori, DrToshio Inui and Dr Kentaro Kubo.
Figure 2: Whole body hyperthermia

This equipment can heat tors within the patient’s body with a special heat control system.
Clinical study of fi rst-generation GMAF for can and HIV treatment
Clinical trials using GMAF in patients with metastatic breast can7, prostate can8, and metastatic colorectal can9 have been conducted. Can did not recur over a four to seven year period in all subjects administrated weekly doses of 100 ng of GMAF for 7 to 19 weeks; a result that took everyone by surprise. However, there are some problems with these clinical trials: there were no clear classifications of patients’ histopathological types, grades, and stages, and the curative judgment was based solely on a patient’s N-acetylgalactosaminidase (Nagse) activity, and neither tor markers or cytokine levels were measured, and there was no control group.
There is also an interesting clinical report of HIV treatment using GMAF10. The weekly administration of 100 ng of GMAF to 15 non-anemic HIV-infected patients showed that the number of CD4+ cells increased to normal levels within 6 weeks, and were maintained for the entire 7 years after GMAF therapy, while the number of CD8+ cells decreased to normal levels, and the amount of HIV-1 RNA and p24 antigen were detectable in these patients blood stream. It is believed that this positive effect resulted from GMAF-activated machages phagocytosizing and destroying the HIV virus. GMAF was active in the monocytes/machages isolated from AIDS patients11.
Pharmaceutical development of first-generation GMAF for immun_o_therapy
There is little or no research being conducted on the pharmaceutical development of GMAF as a biological drug. The pharmaceutical development of GMAF with its high homogeneity and purity from human serum is very difficult owing to its parent Gic pro having six major subtypes, namely homodimer or heterodimer of 1f, 1s, and 2, each with a different sugar moiety (Fig. 3). In order to overcome this problem, Dr Yamamoto manufactured the GMAF mimic glycoprotein using a baculovirus expression system. Dr Giovanna Ghirlanda and colleagues developed a small molecule GMAF constituting 23-mer glycopeptides with a similar sugar chain of GMAF with machage phagocytic activation abilities comparable to GMAF12. We also developed 12-mer GMAF-mimic glycopeptides having their machage phagocytic activation abilities and in-vivo anti-angiogenic activities in the chick embryo’s chorioallantoic membrane (CAM) assay in a joint study with JITSUBO Co., Ltd. We hope that practical pharmaceutical development of GMAF for clinical use will begin soon.
Figure 3: Proposed sugar chain structure of Gic pro subtype
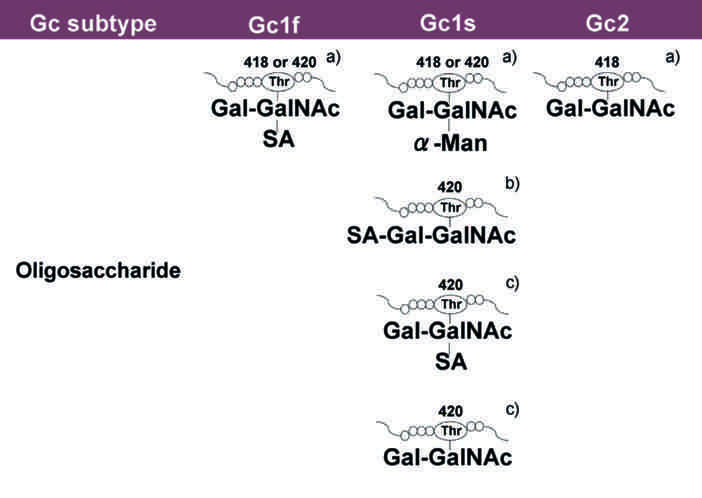
- Sugar structure proposed by Dr Nobuto Yamamoto
- Sugar structure proposed by Dr C.R. Borges with LC-MS/MS analysis
- Sugar structure proposed by Dr. Yoshihiro Uto and Dr. Hitoshi Hori with lectin analysis. GalNAc: N-acetylgalactosamine; Gal: galactose; SA: sialic acid; α-Man: α-mannose
Figure 4: The treatment of patients with GMAF using blood donated by volunteers
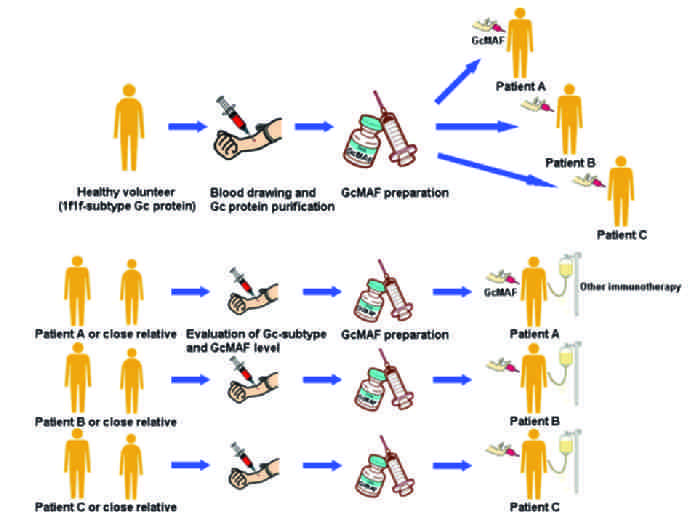
- First-generation GMAF is prepared from the serum of a healthy volunteer having 1f1f-subtype Gic pro. This GMAF is then used to treat unspecified patients
- Second-generation GMAF is prepared from the serum of the can patient or his or her close relatives and used to treat that particular patient specifically
Pharmaceutical development and clinical study of second-generation GMAF for immun_o_therapy
The first-generation GMAF were prepared by artificial enzymatic treatments from 1f1fsubtype Gic pro. First-generation GMAF treatment is not personal and does not consider each patient’s immune status, possibly caused by differences of Gic pro types, GMAF level, and immune state of each subject (Fig, 4A). Accordingly, we propose second-generation GMAF, which considers the patient’s individual immune status. Our second-generation GMAF-based immun_o_therapy (Fig. 4B) is characterized as; 1) the artificial exogeneous preparation of GMAF using a sample of the patient’s serum or serum from his or her close relatives, 2) examination of patient’s GMAF level and immunity status before GMAF treatment, 3) the optimized GMAF preparation is administered to the can patient.
We hypothesized that the GMAF precursor (preGMAF) — the full Gic pro, only lacking the galactosyl moiety — could be converted to GMAF in vivo by processing with constitutive sialidase. We recently found that 1f1f-subtype of preGMAF, as well as GMAF itself, could be used as an effective precursor-type machage activator in vivo13. These results make it clear that β-galactosidase-treatment is a common modification for all subtypes of Gic pro to prepare GMAF in vivo. Morita Pharmaceutical Ind., Ltd. is currently developing preGMAF for preclinical trials and towards clinical application.
At the end of April 2011, Saisei Mirai started treating can patients using second generation GMAF machage activation therapy produced from human serum. As at March 2012, Saisei Mirai have 137 can patients registered as using GMAF. We also plan to conduct a comparative clinical study to clarify the efficacy of several combination therapies of different Gic pro subtypes, different content of GMAF, and different machage status to find the relationship between each combination therapy and the curative effect of human serum including GMAF. We aim to determine the optimal condition of the machage activation therapy from the results of these clinical and analytical studies. Furthermore, secondgeneration therapy is now being used for HIV and also HCV patients at Kobe Saisei Mirai Clinic in Kobe, Japan.
Figure 5: Saisei Mirai Cell Processing Center (CPC)

- Cell culture room in CPC. Each room has an air lock. To prevent contamination of outside air, dust and microbes, each room is maintained at a set room pressure. The conditions in each room and important equipment is monitored 24 hour a day.
- Treatment of cell culture in the safety cabinet. The worker wears special clothes and shoes. We treat cells under aseptic conditions and observe the condition of the culture every day under a microscope.
- The cell-banking system introduced with liquid nitrogen tank. Cells such as T Lym from the patient are kept for long periods for use in the future.
Hyper T/NK cell Therapy
In a previous study, tor-infiltrating Lym (TIL) have been reported to be effective in experimental and clinical research of advanced can14,15. TIL recognize specific antigens expressed by autologous tor cells. However, this specific antigenicity is too low to achieve a high degree of antigenicity in therapeutic use. In order to solve this problem, Sekine and colleagues developed a feasible method to obtain large numbers of activated or effective T Lym 16,17. Cultivation of peripheral blood Lym with immobilized anti-CD3 monoclonal antibody and human recombinant interleukin-2 induces a rapid and massive proliferation of T Lym and greatly augments their cytotoxic activity. Administration of these expanded TILs demonstrates clinical activity in some patients with several types of can, making it a useful adoptive immun_o_therapy.
We developed a cultivation method with autologous plasma from patients with specific antibodies for membrane antigens of natural killer (NK) cells based on Sekine’s techniques and our own clinical results. Briefly, peripheral blood Lym are cultivated with immobilized anti-CD3 monoclonal antibody and human recombinant interleukin-2 as per Sekine’s method, adding several matrix proteins such as fibronectin in plasma or immobilized monoclonal antibody such as CD161 for NK cells18. This cultivation method is used not only to obtain activated T Lym, but also ‘Hyper T cells’ and NK cells. Hyper T cells, a name we coined, are unique immature multipotent T cells with various capabilities. Populations of these cells deplete as we age. In particular, a significant decline in number is evidnent in people over 50 years of age. Hyper T cells have a broader specificity for antigens expressed by autologous tor cells, are able to proliferate and maintain their activity for long periods in vivo19. Therefore, expansion of Hyper T cells has the potential to be a suitable and important factor of adoptive immun_o_therapy against can. NK cells are a unique subset of Lym, distinct from T Lym. They contribute to essential IM systems such as host antimicrobial and antitor immunity without requirement for prior IM sensitization of the host20. NK cells are promising effector cells for immun_o_therapy against can. Three cell types T lLym, Hyper T cells, and NK cells, are cultured simultaneously. Methods to expand one cell type have been developed by several researchers. However, established methods of cultivation are problematic with respect to cost, complexity and safety, such as the risks of using biological tissue of animal or human origin including human serum albumin. Therefore, in spite of its possible effectiveness, it has not been entirely accepted in general medi_cal practice. We developed a simple method of simultaneously culturing — T lymphocytes, Hyper T cells, and NK cells — which together seem to be important for an effective IM therapy. Another advantage of our method is free of animal and human material. Each cell is shown to be effective in can, but many problems are also overlooked. For example, it is said that NK cells have difficulty permeating deep into the tor tissue. Accordingly, it is necessary for NK cells to attack a tor tissue after making a tor is reduced in size by T Lym and Hyper T Lym. Such a sensible strategy makes it an effective IM therapy. Using these cells in combination allows the advantage of one cell type compensate for the disadvantage of using the other alone — their synergistic actions contribute to the eradication of tor cells.
Actually, we tested this combination therapy with systemically administered T Lym, Hyper T cells and NK cells in more than 100 can patients. This method can be of benefit to patients and is a promising immun_o_therapy. We expect that the described immun_o_therapy will play a central role in future treatments against certain human cans, both alone and in combination with other therapies such as GMAF and/or hyperthermia treatment.
Cell Processing Center
Saisei Mirai Cell Processing Center (CPC) is a special clean facility for cell culture and preparation of medicine for the patient (Fig 5). To maintain safety and high quality, only medi_cal specialists with knowledge and skills for cell-culture work are employed at the CPC. In our tissue-engineered products, such as GMAF, Hyper T NK cells, and can vac_cines are manufactured.
References
- Mellman, I. et al. Can immun_o_therapy comes of age. Nature 480, 480–489 (2011)
- Yamamoto, N. et al. Vitamin D3 binding protein (group-specific component) is a precursor for the machage-activating signal factor from lysophosphatidylcholine-treated Lym. Proc. Natl. Acad. Sci. USA 88, 8539–8543 (1991)
- Yamamoto, N. et al. Conversion of vitamin D3 binding protein (groupspecific component) to a macrophageactivating factor by the stepwise action of β-galactosidase of B-cells and sialidase of T-cells. J. Immunol. 151, 2794–2802 (1993)
- Mohamad, S.B. et al. Preparation of Gic pro-derived macrophage activating factor (GMAF) and its structural characterization and biological activities. Antican Res. 22, 4297–4300 (2002)
- Kisker, O. et al. Vitamin D binding protein-machage-activating factor (DBP-maf) inhibits angiogenesis and tor growth in mice. Neoplasia 5, 32–40 (2003)
- Pacini, S. et al. Effects of vitamin D-binding protein-derived machage-activating factor on human breast can cells. Antican Res. 32, 45–52 (2012)
- Yamamoto, N. et al. Immun_o_therapy of metastatic breast can patients with vitamin D-binding proteinderived machage activating factor (GMAF). Int. J. Can 122, 461–467 (2008)
- Yamamoto, N. et al. Immun_o_therapy for prostate can with Gic Pro derived machage-activating factor, GMAF. Transl. Oncol. 1, 65–72 (2008)
- Yamamoto, N. et al. Immun_o_therapy of metastatic colorectal can with vitamin D-binding protein-derived machage activating factor, GMAF. Can Immunol. Immunother. 57, 1007–1016 (2008)
- Yamamoto, N. et al. Immun_o_therapy of HIV-infected patients with Gic pro-derived machage activating factor (GMAF). J. Med. Virol. 81, 16–21 (2009)
- Yamamoto, N. et al. Structural modification of serum vitamin D3-binding protein and immun_osuppression in AIDS patients. AIDS Res. Hum. Retroviruses 11,1373–1378 (1995)
- Bogani, F. et al. A designed glycoprotein analogue of Gc-MAF exhibits native-like phagocytic activity. J. Am. Chem. Soc. 128, 7142–7143 (2006)
- Uto, Y. et al. Effect of the Gc-derived machage-activating factor precursor (preGMAF) on phagocytic activation of mouse peritoneal machages. Antican Res. 31, 2489–2492 (2011)
- Rosenberg, S.A. et al. A new approach to the adoptive immun_otherapy of can with tor-infiltrating Lym. Science 233, 1318–1321 (1986)
- Rosenberg, S.A. et al. A progress report on the treatment of 157 patients with advanced can using lymphokineactivated killer cells and interleukin-2 or high-dose interleukin-2 alone. N. Eng. J. Med. 316, 889–897 (1987)
- Yamazaki, T. et al. Characterization of immobilized anti-CD3 antibody activated T Lym for use in adoptive immun_o_therapy of patients with brain tors. NEUROL Med. Chir. (Tokyo) 32, 255–261 (1992)
- Sekine, T. et al. A feasible method for expansion of peripheral blood Lym by culture with immobilized anti-CD3 monoclonal antibody and interleukin-2 for use in adoptive immun_o_therapy of can patients. Biomed. and Pharamocother. 47, 73–78 (1993)
- Pozo, D. et al. CD161 (Human NKRP1A) signaling in NK cells involves the activation of acid spingomyelinase. J. Immunol. 176, 2397–2406 (2006)
- Utsuyama, M. et al. Differential age-change in the numbers of CD4+CD45RA+ and CD4+CD29+ T cell subsets in human peripheral blood. Mech. Ageing. Dev. 63, 57–68 (1992)
- Ljunggren, H.G. et al. Prospects for the use of NK cells in immun_o_therapy of human can. Immunology 7, 329–339 (2007)
Case Report: A Breast Can Patient Treated with GMAF, Sonodynamic Therapy and Hormone Therapy
Abstract
Background: Gic pro-derived machage-activating factor (GMAF) occurs naturally in the human body. It has various functions, such as machage activation and anti activities. Recently, immun_o_therapy has become an attractive new strategy in the treatment of can. GMAF-based immun_o_therapy can be combined with many other therapies. Sonodynamic therapy (SDT) using low-intensity ultrasound is a novel therapeutic modality. Ultrasound has been demonstrated to activate a number of sonosensitive agents allowing for the possibility of non-invasive targeted treatment for both superficial and deep-seated tors. The current case study demonstrates that GMAF and SDT can be used in combination with conventional therapies in patients with metastatic can, especially where treatment options are limited due to factors such as toxicity. This case study also suggests a new concept of can treatment using local destruction of can tissue, in this case conducted with SDT, to be used in combination with GMAF as a systemic treatment.
Imute has become an attractive new strategy in the treatment of can, due in part to minimal toxicity and an excellent safety profile when compared to conventional therapies such as che_motherapy and radiation.
Gic pro-derived machage-activating factor (GMAF). Machages are important phagocytic cells that internalize and destroy pathogens and release cytokines. In addition, machages are known to play critical roles in anti immunity. They can infiltrate into tors and are found in most tor sites. GMAF was first developed by Dr. Nobuto Yamamoto in 1991 (1), suggesting that it can be used as an important immun_o_therapy for can treatment (2-7). In previous studies, Gic pro (1f1f subtype) was isolated from human serum using an affinity column modified with 25-hydroxyvitamin D3. GMAF was then prepared from this isolated Gic pro by an artificial enzymatic method (8). The process of separating Gic pro from serum leads to a much lower concentration, stability and activity of the final GMAF that is produced. Additionally, re-use of the affinity column must be avoided because of cross-contamination between different serum samples. To overcome the problems associated with isolating Gic pro from serum in the production of GMAF, we prepared de-galactosylated/desialylated human serum, which we coined serum GMAF or second-generation GMAF. Second generation GMAF has a higher concentration (1,500 ng/0.5 ml) and longer stability because of this new manufacturing process, demonstrating that the 25-OH vitamin D affinity column is not necessary (Table I). Saisei Mirai developed a second-generation GMAF in 2011, in collaboration with Dr. Hitoshi Hori and Dr. Yoshihiro Uto at Tokushima University (9). In addition to the higher concentration and stability, it has several other important properties, which include increasing phagocytic activity of machages (10), superoxide radical generation (11), anti-angiogenic effects (12), and anti effects (13). Increases in the number of monocytes and monocyte percentage in the blood have been observed in many patients during GMAF therapy. In addition, serum GMAF has been shown to increase the maturation of dendritic cells in vitro (unpublished data). By March 2014, Saisei Mirai will have treated more than 1,000 patients with GMAF, both with and without conventional therapies, proving its safety as a therapy.
Comparison between first and second generation GiC pro-derived machage-activating factor
Second generation (9, 10)
Developed by Saisei Mirai and the University of Tokushima in 2011
High concentration (1500 ng/0.5ml, 1 dose)
Significantly higher stability and machage-activating activity
New patented production process
Sonodynamic therapy (SDT). There is an ever-increasing amount of data showing that SDT, which refers to the use of low-intensity ultrasound with a sonosensitizer, can be used to produce free radical oxygen (14) to selectively destroy can cells (15, 16, 17). The concept of SDT consists of introducing a substance into the body that preferentially accumulates in can cells (15). This substance is then activated by ultrasound vibration instead of light stimulation, in contrast to traditional photodynamic therapy using a light-activated sensitizer. Since ultrasound is capable of passing completely through the body, the concept of destroying can cells without using damaging invasive procedures becomes possible. It also has the ability to destroy metastases in most places in the body, making it a very versatile and important therapy. SDT is considered to be a promising new modality for can treatment, without causing serious side-effects. Numerous sensitizers with various properties are being developed in a number of countries around the world. Recently, new sonosensitizers which only respond to ultrasound have been developed in Japan (14). This allows for the treatment of patients without light toxicity, which can be sustained during exposure to natural sunlight while the sensitizer remains in the body.
Ozone autohemotherapy and hyperbaric oxygen therapy. Tor hypoxia, in which the tor is deprived of an adequate oxygen supply, is a well-recognized factor in can treatment resistance to che_motherapy and radiotherapy as well as SDT, which requires production of oxygen-free radicals in order to be effective (15). Therefore any method of increasing oxygen supply within the tor environment should increase the efficacy of SDT (15). Ozone therapy is a medi_cal treatment that is used to increase the amount of oxygen in the blood. It is achieved by ozonating the patient's own blood outside the body and injecting it back into the body within a relatively short amount of time. Hyperbaric oxygen therapy is the medi_cal use of oxygen at levels higher than atmospheric pressure. The equipment used for hyperbaric oxygen therapy consists of a pressure chamber and a means of delivering pure oxygen. In clinical situations, SDT is usually combined with ozone autohemotherapy to improve local hypoxia within the tor environment.
Case Report
A 55-year-old female was diagnosed with breast can (left side, with skin invasion) in August 2009. She was treated by lumpectomy, with no che_motherapy or radiotherapy. She refused further standard treatment after the operation. The tor was estrogen (ER)-, progesterone (PR)-, and receptor (HER2)-positive. In October 2011, she noticed a right axillary tor. At that time no treatments had been undertaken. The tor kept growing and tor markers increased. In July 2012, a needle aspiration biopsy was carried-out to confirm the recurrence of the tor. The patient still refused standard treatment and underwent hyperthermia (total 24 times with Thermotron RF-8) and i.v. high dose vitamin C (total 10 times). She presented at our Clinic in January 2013. Her symptoms at presentation were a cough, back pain and severe swelling of the right arm (edema), and her pathological findings were invasive ductal carcinoma, N0 (no nodes were involved), negative surgical margin, grade 3, ER+, PR+, HER2+. Chest positron emission tomography and computed tomography (PET CT) on 6th June 2013 (Figure 1) showed a right axillary tor, spinal metastases, intrapleural nodular tor and right pleural effusion.
Second-generation high-dose GMAF was administered at 0.5 ml, two times a week intramuscularly, for a total of 21 times. Sensitizers for SDT were modified in chlorin e6 at 25 mg i.v., and 5-at 10 mg/kg orally. A total of 19 treatments of SDT were conducted from June to September 2013. (Aromasin) aromatase inhibitor was also given to the patient at a dose of 25 mg/day orally.
By the beginning of October 2013 the patient showed dramatic improvement of symptoms such as cough, back pain and edema of the right hand from the combination therapy with SDT, GMAF and hormone therapy. Her axillary tor (Figure 1A) decreased in size and disappeared completely.
Figure 1
- Chest tomography of a 55-year-old female patient on 6th June 2013 showing a right axillary tor
- spinal metastases
- intrapleural nodular tor and right pleural effusion
Figure 2
- Chest positron emission tomography and computed tomography (PET CT) horizontal plane of a 55-year-old female patient 6th June 2013 showing lung pleural effusion and intrapleural nodular tor before treatment with sonodynamic therapy SDT
- Chest CT horizontal plane on 9th September 2013 showing the complete disappearance of the lung pleural effusion and nodular shadow in the right lung after treatment with SDT and hormonal therapy
Chest PET CT on 6th June 2013 (Figure 2A) showed lung pleural effusion and intrapleural nodular tor before treatment with SDT. A later chest CT on 9th September 2013 (Figure 2B) showed complete disappearance of the pleural effusion and intra-pleural nodular tor in the right lung after treatment with SDT, GMAF and hormone therapy.
There was a significant increase in monocyte percentage and monocyte number (Figure 3) and a rapid decrease in tor markers (Figure 4) during the treatment period from January 2013 to October 2013. There were no serious side-effects from the treatments except slight joint pain from the hormonal therapy with aromatase inhibitor.
Discussion
Can is a broad group of diseases involving deregulated cell growth, forming malignant tors which invade nearby parts of the body and can metastasize and spread to more distant parts. Therefore it is important to destroy local can tissue using therapies with minimal side-effects, whilst at the same time stimulating the IM system and allowing antigen-presenting cells display tor-associated antigens to helper T-cells. This basic concept of recognizing tor-associated antigens and teaching the IM system to attack can cells inside the body is a very similar idea to that of a can vaccine, even though a can vaccine itself is always created outside the body. Immun_o_therapy is both a local and a systemic therapy which can be used in combination with local tor destruction in the case of large tor burden.
Figure 3
Change in blood monocyte percentage and monocyte number of 55-year-old female patient during Gic pro-derived machage-activating factor (GMAF) therapy. The patient's monocyte number rose during high-dose GMAF treatment, indicating a good response to the therapy
Figure 4
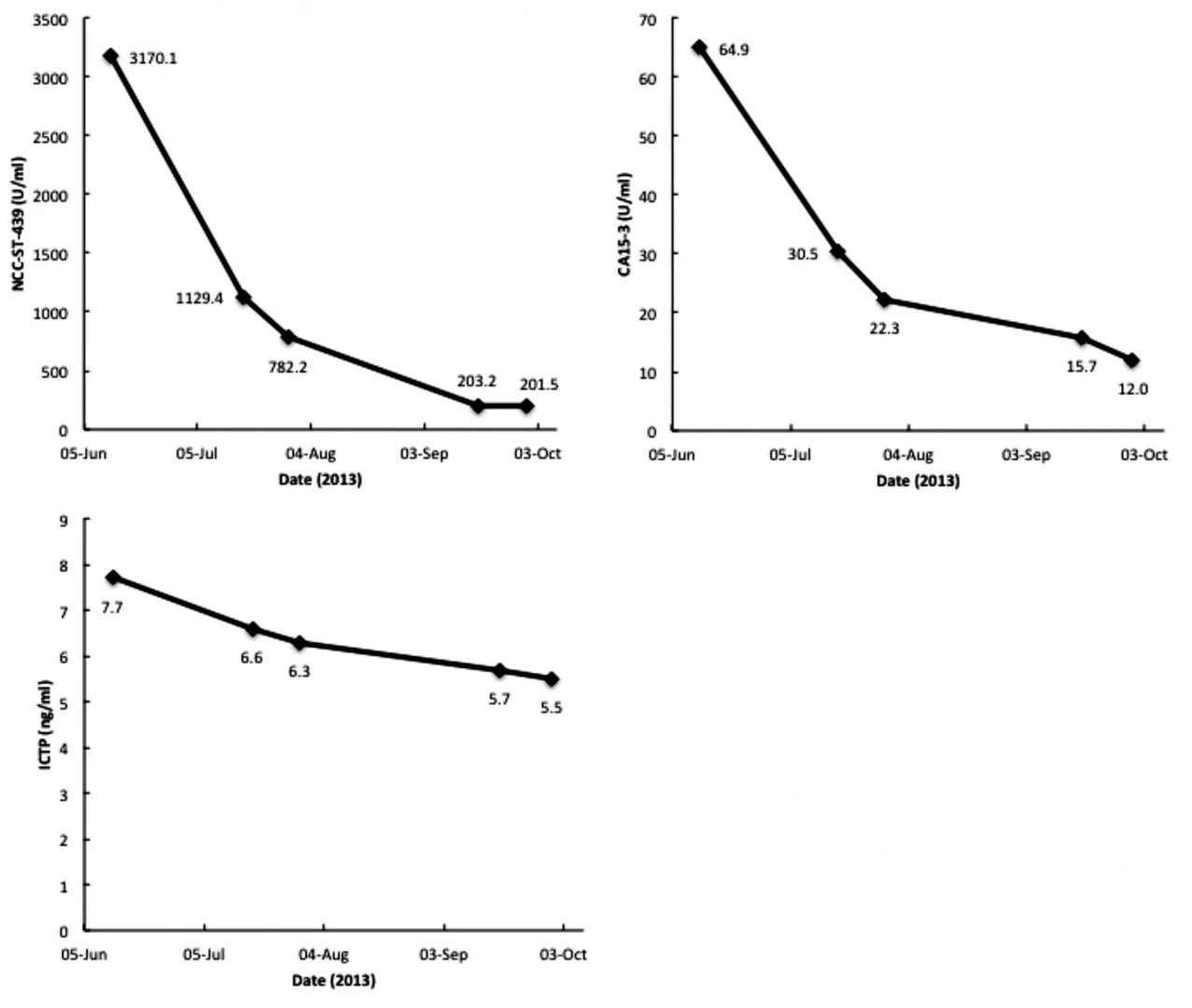
Time course of tor markers, nation can center-stomach-439 (NCC-ST-439), carbohydrate antigen 15-3 (CA15-3), and carboxy terminal telopeptide of type I collagen (ICTP). The patient's tor markers rapidly decreased during the treatment period.
We highlight this case of a patient with terminal breast can having had good effects from SDT, GMAF and hormonal therapy. It suggests that SDT and GMAF can be used in combination with standard treatments, in particular targeted-therapies, with minimal toxicity and without negative effects on the IM system, to achieve better outcomes for patients with can. SDT, GMAF and hormonal therapies are non-invasive, well-tolerated treatments that may be capable of controlling tor progression by working synergistically. Furthermore, SDT and GMAF may be capable of controlling tor progression by inducing direct inflammatory necrosis inside tors, producing antitor immunity via antigen-presenting cells to prevent IM escape in a variety of deep and superficial tors.
Utilizing these new approaches gives those of us who have been treating can good weapons that kill can cells selectively, efficiently, and by non-toxic and painless means. We are planning to further refine and improve our protocols with SDT and GMAF.
References
- Yamamoto N, Homma S: Vitamin D3-binding protein (group-specific component) is a precursor for the machage-activating signal factor from lysophosphatidylcholine-treated Lym. Proc Natl Acad Sci USA 88: 8539-8543, 1991
- Yamamoto N, Suyama H, Yamamoto N, Ushijima N: Immun_o_therapy of metastatic breast can patients with vitamin D-binding protein-derived machage-activating factor (GMAF). Int J Can_cer 122: 461-467, 2008
- Pacini S, Punzi T, Morucci G, Gulisano M, Ruggiero M: Effects of vitamin D-binding protein-derived machage-activating factor on human breast can cells. Antican Res 32: 45-52, 2012
- Yamamoto N, Suyama H, Yamamoto N: Immun_o_therapy for prostate can with Gic Pro-derived machage-activating factor, GMAF. Transl Oncol 1: 65-72, 2008
- Yamamoto N, Suyama H, Nakazato H, Yamamoto N, Koga Y: Immun_o_therapy of metastatic colorectal can with vitamin D-binding protein-derived macrophage activating factor, GMAF. Can Immunol Immunother 57: 1007-1016, 2008
- Yamamoto N, Ushijima N, Koga Y: Immun_o_therapy of HIV-infected patients with Gic pro-derived machage-activating factor (GMAF). J Med Virol 81: 16-21, 2009
- Yamamoto N, Naraparaju VR, Srinivasula SM: Structural modification of serum vitamin D3-binding protein and immun_osuppression in AIDS patients. AIDS Res Hum Retroviruses 11: 1373-1378, 1995
- Yamamoto N, Kumashiro R: Conversion of vitamin D3-binding protein (group-specific component) to a machage-activating factor by the stepwise action of beta-galactosidase of B-cells and sialidase of T-cells. J Immunol 151: 2794-2802, 1993
- Uto Y, Hori H, Kubo K, Ichihashi M, Sakamoto N, Mette M, Inui T: GMAF: our next-generation immun_o_therapy. Nature 485: S67-S70, 2012
- Kuchiike D, Uto Y, Mukai H, Ishiyama N, Abe C, Tanaka D, Kawai T, Kubo K, Mette M, Inui T, Endo Y, Hori H: Degalactosylated/desialylated human serum containing GMAF induces machage phagocytic activity and in vivo anti activity. Antican Res 33: 2881-2885, 2013
- Mohamad SB, Nagasawa H, Uto Y, Hori H: Preparation of Gic pro-derived machage activating factor (GMAF) and its structural characterization and biological activities. Antican Res 22: 4297-4300, 2002
- Kisker O, Onizuka S, Becker CM, Fannon M, Flynn E, D'Amato R, Zetter B, Folkman J, Ray R, Swamy N, Pirie-Shepherd S: Vitamin D binding protein-machage-activating factor (DBP-maf) inhibits angiogenesis and tor growth in mice. Neoplasia 5: 32-40, 2003
- Inui T, Kuchiike D, Kubo K, Mette M, Uto Y, Hori H, Sakamoto N: Clinical experience of integrative can immun_o_therapy with GMAF. Antican Res 33: 2917-2919, 2013
- Kuroki M, Hachimine K, Abe H, Shibaguchi H, Kuroki M, Maekawa S, Yanagisawa J, Kinugasa T, Tanaka T, Yamashita Y: Sonodynamic therapy of can using novel sonosensitizers. Antican Res 27: 3673-3678, 2007
- Kenyon J, Fuller R: Outcome measures following sonodynamic photodynamic therapy: a case series. Current Drug Therapy 6: 12-16, 2011
- Huang Z: A review of progress in clinical photodynamic. Ther Technol Can Res Treat 4: 283-293, 2005
- Roberts WG, Hasan T: Tor-secreted vascular permeability factor/vascular endothelial growth factor influences photosensitizer uptake. Can Res 53: 153-157, 1993




